 | |
| Names | |
|---|---|
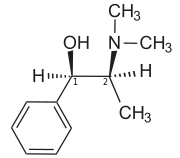
| IUPAC name
(1R,2S)-2-(Dimethylamino)-1-phenyl-1-propanol | |
| Other names
N-Methyl-L-ephedrine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.203 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H17NO | |
| Molar mass | 179.263 g·mol−1 |
| Melting point | 87 to 87.5 °C (188.6 to 189.5 °F; 360.1 to 360.6 K)[1] 192 °C (HCl)[1] |
| Readily soluble[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
N-Methylephedrine is a derivative of ephedrine. It has been isolated from Ephedra distachya.[2]
In organic chemistry, N-methylephedrine is used as a resolving agent and as a precursor to chiral supporting electrolytes, phase-transfer catalysts, and reducing agents.[3] Pharmacologically, N-methylephedrine is a non-selective adrenergic receptor agonist.[4]
Just like ephedrine, it may have abuse potential . N-methylephedrine is one of the four constituents of BRON, a Japanese OTC cough medicine with reports of abuse.[5] Addiction liability of BRON was attributed primarily to the Codeine component.
See also
References
- 1 2 3 Merck Index, 11th Edition, 5987
- ↑ Smith (1927). "CCLXX. l-Methylephedrine, an alkaloid from Ephedra species". J. Chem. Soc.: 2056–2059. doi:10.1039/jr9270002056.
- ↑ (1R,2S)-(−)-N-Methylephedrine at Sigma-Aldrich
- ↑ "KEGG DRUG: dl-Methylephedrine hydrochloride". www.genome.jp. Retrieved 2023-01-12.
- ↑ Jun, Ishigooka; Yoshiko, Yoshida; Mitsukuni, Murasaki (1991-01-01). "Abuse of "BRON": A Japanese OTC cough suppressant solution containing methylephedrine, codeine, caffeine and chlorpheniramine". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 15 (4): 513–521. doi:10.1016/0278-5846(91)90026-W. ISSN 0278-5846. PMID 1749828. S2CID 53187238.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.