| Owlet moths | |
|---|---|
_Ear_Moth_(Amphipoea_oculea)_(20089467344).jpg.webp) | |
| Amphipoea oculea | |
.jpg.webp) | |
| Panthea coenobita | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Lepidoptera |
| Superfamily: | Noctuoidea |
| Family: | Noctuidae Latreille, 1809 |
| Type species | |
| Noctua pronuba | |
| Subfamilies | |
| |
| Diversity | |
| About 11,772 species | |
The Noctuidae, commonly known as owlet moths, cutworms or armyworms, are a family of moths. They are considered the most controversial family in the superfamily Noctuoidea because many of the clades are constantly changing, along with the other families of the Noctuoidea.[1][2][3] It was considered the largest family in Lepidoptera for a long time, but after regrouping Lymantriinae, Catocalinae and Calpinae within the family Erebidae, the latter holds this title now.[4] Currently, Noctuidae is the second largest family in Noctuoidea, with about 1,089 genera and 11,772 species.[5] This classification is still contingent, as more changes continue to appear between Noctuidae and Erebidae.
Description
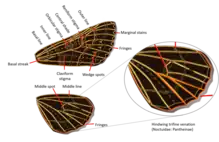
Adult: Most noctuid adults have wings with a variety of shades of browns, grays, and other varied shades and colourations but some subfamilies, such as Acronictinae and Agaristinae, are very colorful, especially those from tropical regions (e.g. Baorisa hieroglyphica). They are characterized by a structure in the metathorax called the nodular sclerite or epaulette, which separates the tympanum and the conjunctiva in the tympanal organ. It functions to keep parasites (Acari) out of the tympanal cavity. Another characteristic in this group is trifine hindwing venation, by reduction or absence of the second medial vein (M2).[6]
Markings present on the wings of noctuid adults can be helpful in distinguishing species. From the basal location to the outer edge (proximal to distal) on the forewing, there is a claviform (club-shaped) stigma, horizontally oriented with the thicker end closer to the wing's outer edge, located posterior to a discal (round) stigma.[7] These are followed distally by a reniform (kidney-shaped) stigma,[8] which is typically oriented with its concave side facing the wing's outer edge. It is often not possible to discern all of the stigmata on all specimens or species.[7] Crossbands or crosslines may be present, oriented longitudinally from the leading to the trailing edge of the wing.[8]
Larva: Commonly green or brown; some species present bright colors, such as the camphorweed cucullia moth (Cucullia alfarata). Most are pudgy and smooth with rounded short heads and few setae, but there are some exceptions in some subfamilies (e.g. Acronictinae and Pantheinae).[9]
Pupa: The pupae most often range from shiny brown to dark brown. When they newly pupate they are bright brownish orange, but after a few days start to get darker.
Eggs: Vary in colors, but all have a spherical shape.
Etymology
The word Noctuidae is derived from the name of the type genus Noctua, which is the Latin name for the little owl, and the patronymic suffix -idae used typically to form taxonomic family names in animals.[10]
The common name "owlet" originally means a small or young owl. The names "armyworms" and "cutworms" are based on the behavior of the larvae of this group, which can occur in destructive swarms and cut the stems of plants.[11]
Ecology
Distribution and diversity
.jpg.webp)
This family is cosmopolitan and can be found worldwide except in the Antarctic region. Some species, such as the setaceous Hebrew character (Xestia c-nigrum), can be found in the Arctic Circle, specifically in the Yukon territory of western Canada, with an elevation 1,702 m above sea level, where the temperature fluctuates between 23/-25 °C (73/-13 °F).[12] Many species of dart moths have been recorded in elevations as high as 4,000 m above sea level (e.g. Xestia elisabetha).[13] Among the places where the number of species has been counted are North America and northern Mexico, with about 2,522 species. 1,576 species are found in Europe, while the other species are distributed worldwide.[3][14][15][16][17]
Mutualism
.jpg.webp)
Members of Noctuidae, like other butterflies and moths, perform an important role in plant pollination. Some species have developed a stronger connection with their host plants. For example, the lychnis moth (Hadena bicruris) has a strange mutualistic relationship with pink plants or carnation plants (Caryophyllaceae), in that larvae feed on the plant while the adults pollinate the flowers.[18]
.jpg.webp)
Food guilds
Herbivory: Caterpillars of most Noctuidae feed on plants; some feed on poisonous plants and are unaffected by their chemical defences; for example, the splendid brocade moth (Lacanobia splendens) feeds on cowbane (Cicuta virosa), a plant that is notoriously toxic to vertebrates.[19]
Predation and cannibalism: During the larval stage, some cutworms readily feed on other insects. One such species is the shivering pinion (Lithophane querquera), whose larvae commonly feed on other lepidopteran larvae.[20] Moreover, many noctuid larvae, such as those of the fall armyworm (Spodoptera frugiperda) and of genera such as Heliothis and Helicoverpa, aggressively eat their siblings and often other species of caterpillar.[21]
Nectarivory and puddling: Like many Lepidoptera, many species of adult Noctuidae visit flowers for their nectar. They also seek other liquid food resources such as plant juices, honeydew, dung, urea and mud, among others.[22]
As is common in members of the order Lepidoptera, courtship in many Noctuidae includes a set of movements in which the female evaluates the male's reproductive fitness.[22]
Most noctuid moths produce pheromones that attract the opposite sex. Female pheromones that attract males occur widely and have long been studied, but the study of male pheromones has further to go.[22][23][24]
Reproduction
.jpg.webp)
Noctuid moths commonly begin the reproductive season from spring to fall, and mostly are multivoltine, such as the eastern panthea moth (Panthea furcilla), which reproduces over the year.[25] Nevertheless, some species have just one brood of offspring (univoltine); among the best known is the lesser yellow underwing (Noctua comes).[25]
Defense

This group has a wide range of both chemical and physical defenses. Among the chemical defenses three types stand out. First, the pyrrolizidine alkaloid sequestration usually present in Arctiinae is also found in a few species of noctuids, including the Spanish moth (Xanthopastis timais).[26] Another chemical defense is formic acid production, which was thought to be present only in Notodontidae, but later was found in caterpillars of Trachosea champa.[27] Finally, the last type of chemical defense is regurgitation of plant compounds, often used by many insects, but the cabbage palm caterpillar (Litoprosopus futilis) produces a toxin called toluquinone that deters predators.[28]
On the other hand, the main physical defense in caterpillars and adults alike is mimicry. Most owlet moths have drab colors with a variety of patterns suitable to camouflage their bodies.[25] The second physical defense consists in thousands of secondary setae that surround the body. The subfamilies that present this mechanism are Pantheinae and Acronictinae. The third is aposematism, represented by species of Cucullinae.[25] Finally, all adults have another mechanism for defense: a tympanal organ available to hear the echolocation spread out by bats, so the moths can avoid them.[29]
Human importance
.jpg.webp)
Agriculture
Many species of owlet moths are considered an agricultural problem around the world. Their larvae are typically known as "cutworms" or "armyworms" due to enormous swarms that destroy crops, orchards and gardens every year. The Old World bollworm (Helicoverpa armigera) produces losses in agriculture every year that exceed US$2 billion.[30] Additionally, the variegated cutworm (Peridroma saucia) is described by many as one of the most damaging pests to vegetables.[31]
In West Africa, species including Busseola fusca, Heliocheilus albipunctella, Sesamia calamistis, Helicoverpa armigera, and Spodoptera exempta are major pests of staple crops such as pearl millet, sorghum, and maize.[32]
Systematics
Since molecular analysis began to play a larger role in systematics, the structure of many Lepidoptera groups has been changing and Noctuidae is not an exception. Most recent studies have shown that Noctuidae sensu stricto is a monophyletic group, mainly based on trifine venation. Some clades within Noctuidae sensu lato have yet to be studied. This taxonomic division represents the subfamilies, tribes and subtribes considered so far.[1][14][33][34]
- Family Noctuidae Latreille, 1809
- Subfamily Acontiinae Guenée, 1841
- Tribe Acontiini Guenée, 1841
- Tribe Armadini
- Tribe Chamaecleini
- Subfamily Acronictinae Harris, 1841
- Subfamily Aediinae
- Subfamily Agaristinae Boisduval, 1833
- Subfamily Amphipyrinae Guenée, 1837
- Tribe Amphipyrini Guenée, 1837
- Tribe Psaphidini Grote, 1896
- Subtribe Feraliina Poole, 1995
- Subtribe Nocloina Poole, 1995
- Subtribe Psaphidina Grote, 1896
- Subtribe Triocnemidina Poole, 1995
- Subfamily Bagisarinae Crumb, 1956
- Subfamily Balsinae Grote, 1896
- Subfamily Bryophilinae Guenée, 1852
- Subfamily Cobubathinae Wagner & Keegan, 2021
- Subfamily Condicinae Poole, 1995
- Tribe Condicini Poole, 1995
- Tribe Leuconyctini Poole, 1995
- Subfamily Cropiinae Keegan & Wagner, 2021
- Subfamily Cuculliinae Herrich-Schäffer, 1850
- Subfamily Dilobinae
- Subfamily Dyopsinae
- Subfamily Eriopinae Herrich-Schäffer, 1851
- Subfamily Eucocytiinae
- Subfamily Eustrotiinae Grote, 1882
- Subfamily Grotellinae
- Subfamily Heliothinae Boisduval, 1828
- Subfamily Metoponiinae Herrich-Schäffer, 1851
- Tribe Cydosiini Kitching & Rawlins, 1998
- Subfamily Noctuinae Latreille, 1809
- Tribe Actinotiini Beck, 1996
- Tribe Apameini Guenée, 1841
- Tribe Arzamini Grote, 1883
- Tribe Caradrinini Boisduval, 1840
- Subtribe Athetiina Fibiger & Lafontaine, 2005
- Subtribe Caradrinina Boisduval, 1840
- Tribe Dypterygiini Forbes, 1954
- Tribe Elaphriini Beck, 1996
- Tribe Episemini
- Tribe Eriopygini Fibiger & Lafontaine, 2005
- Tribe Glottulini Guenée, 1852
- Tribe Hadenini Guenée, 1837
- Tribe Leucaniini Guenée, 1837
- Tribe Noctuini Latreille, 1809
- Subtribe Agrotina Harris, 1841
- Subtribe Axyliina
- Subtribe Noctuina Latreille, 1809
- Tribe Orthosiini Guenée, 1837
- Tribe Phlogophorini Hampson, 1918
- Tribe Phosphilini Poole, 1995
- Tribe Prodeniini Forbes, 1954
- Tribe Pseudeustrotiini Beck, 1996
- Tribe Tholerini Beck, 1996
- Tribe Xylenini Guenée, 1837
- Subtribe Antitypina Forbes & Franclemont, 1954
- Subtribe Cosmiina Guenée, 1852
- Subtribe Ufeina Crumb, 1956
- Subtribe Xylenina Guenée, 1837
- Subfamily Oncocnemidinae Forbes & Franclemont, 1954
- Subfamily Pantheinae Smith, 1898
- Subfamily Plusiinae Boisduval, 1828
- Tribe Abrostolini Eichlin & Cunningham, 1978
- Tribe Argyrogrammatini Eichlin & Cunningham, 1978
- Tribe Plusiini Boisduval, 1828
- Subtribe Autoplusiina Kitching, 1987
- Subtribe Euchalciina Chou & Lu, 1979
- Subtribe Plusiina Boisduval, 1828
- Subfamily Raphiinae
- Subfamily Stiriinae
Genera with intervening taxonomy not available include:
References
- 1 2 Regier, Jerome C.; Mitter, Charles; Mitter, Kim; Cummings, Michael P.; Bazinet, Adam L.; Hallwachs, Winifred; Janzen, Daniel H.; Zwick, Andreas (1 January 2017). "Further progress on the phylogeny of Noctuoidea (Insecta: Lepidoptera) using an expanded gene sample". Systematic Entomology. 42 (1): 82–93. doi:10.1111/syen.12199. ISSN 1365-3113.
- ↑ Lafontaine, J. Donald; Fibiger, Michael (1 October 2006). "Revised higher classification of the Noctuoidea (Lepidoptera)". The Canadian Entomologist. 138 (5): 610–635. doi:10.4039/n06-012. ISSN 1918-3240. S2CID 86122393.
- 1 2 Michael, Fibiger; Donald, Lafontaine, J.; H., Hacker, Hermann (1 January 2005). A Review of the Higher Classification of the Noctuoidea (Lepidoptera) With Special Reference to the Holarctic Fauna. Beilage zu Band 11: (Notodontidae, Nolidae, Arctiidae, Lymantriidae, Erebidae, Micronoctuidae, and Noctuidae): Gesamtinhaltsverzeichnis Bände 1-10: Indices Bände 1-10. Delta-Druck und Verlag Peks. ISBN 978-3938249017. OCLC 928877801.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Zahiri, Reza; Holloway, Jeremy D.; Kitching, Ian J.; Lafontaine, J. Donald; Mutanen, Marko; Wahlberg, Niklas (1 January 2012). "Molecular phylogenetics of Erebidae (Lepidoptera, Noctuoidea)". Systematic Entomology. 37 (1): 102–124. doi:10.1111/j.1365-3113.2011.00607.x. ISSN 1365-3113. S2CID 84249695.
- ↑ Zhang, Z.-Q., ed. (23 December 2011). "Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness". Zootaxa. Magnolia Press. 3148: 217. ISBN 9781869778491.
- ↑ Fibiger, Michael (2007). The Lepidoptera of Israel. Coronet Books Incorporated. ISBN 9789546422880.
- 1 2 Hudson, G.V. (1898). "I.—The Caradrinina". New Zealand Moths and Butterflies (Macro-Lepidoptera). London: West, Newman & Co. Retrieved 31 July 2022.
- 1 2 Rackham, Tony. "Moths - Glossary". Identify British Moths. Retrieved 31 July 2022.
- ↑ Wagner, David L. (25 April 2010). Caterpillars of Eastern North America: A Guide to Identification and Natural History. Princeton University Press. ISBN 978-1400834143.
- ↑ Speidel, W.; Naumann, C. M. (2004). "A survey of family‐group names in noctuoid moths (Insecta: Lepidoptera)". Systematics and Biodiversity. 2 (2): 191–221. doi:10.1017/S1477200004001409. ISSN 1477-2000. S2CID 85652010.
- ↑ Rice, Marlin E. (1 January 2004). "Armyworm defoliating young corn". Integrated Crop Management News.
- ↑ Lafontaine, J. D.; Wood, D. M. "Butterflies and moths of the Yukon" (PDF). E.H. Strickland Entomological Museum.
- ↑ Gyulai, P.; Ronkay, L.; Saldaitis, A. (4 November 2013). "Two new Xestia Hübner, 1818 species from China (Lepidoptera, Noctuidae)". Zootaxa. 3734 (1): 96–100. doi:10.11646/zootaxa.3734.1.12. ISSN 1175-5334. PMID 25277901. S2CID 21336866.
- 1 2 Schmidt, B. Christian; Lafontaine, J. Donald (19 March 2010). Annotated Check List of the Noctuoidea (Insecta, Lepidoptera) of North America North of Mexico. PenSoft Publishers LTD. ISBN 9789546425355.
- ↑ Schmidt, Bjorn Christian; Lafontaine, J. Donald (24 November 2011). Contributions to the Systematics of New World Macro-moths III. PenSoft Publishers LTD. pp. 1–4. doi:10.3897/zookeys.149.2383. ISBN 9789546426185. PMC 3234404. PMID 22207789.
{{cite book}}:|journal=ignored (help) - ↑ Lafontaine, Donald; Schmidt, Christian (2 June 2013). "Additions and corrections to the check list of the Noctuoidea (Insecta, Lepidoptera) of North America north of Mexico". ZooKeys (264): 227–236. doi:10.3897/zookeys.264.4443. ISSN 1313-2970. PMC 3668382. PMID 23730184.
- ↑ Lafontaine, J. Donald; Schmidt, B. Christian (15 October 2015). "Additions and corrections to the check list of the Noctuoidea (Insecta, Lepidoptera) of North America north of Mexico III". ZooKeys (527): 127–147. doi:10.3897/zookeys.527.6151. ISSN 1313-2989. PMC 4668890. PMID 26692790.
- ↑ Bopp, Sigrun; Gottsberger, Gerhard (1 January 2004). "Importance of Silene latifolia ssp. alba and S. dioica (Caryophyllaceae) as Host Plants of the Parasitic Pollinator Hadena bicruris (Lepidoptera, Noctuidae)". Oikos. 105 (2): 221–228. doi:10.1111/j.0030-1299.2004.12625.x. JSTOR 3548083.
- ↑ Jacobs, Maarten (2005). "Lacanobia splendens, a new species for the Belgian fauna (Lepidoptera: Noctuidae)" (PDF). Phegea. 33 (3): 83–85.
- ↑ Schweitzer, D. F. (1 January 1979). "Predatory behavior in Lithophane querquera and other spring caterpillars". Journal of the Lepidopterists' Society. ISSN 0024-0966.
- ↑ Chapman, Jason W.; Williams, Trevor; Martínez, Ana M.; Cisneros, Juan; Caballero, Primitivo; Cave, Ronald D.; Goulson, Dave (1 January 2000). "Does Cannibalism in Spodoptera frugiperda (Lepidoptera: Noctuidae) Reduce the Risk of Predation?". Behavioral Ecology and Sociobiology. 48 (4): 321–327. doi:10.1007/s002650000237. JSTOR 4601817. S2CID 3947934.
- 1 2 3 Birch, Martin (1 May 1970). "Pre-courtship use of abdominal brushes by the nocturnal moth, Phlogophora meticulosa (L.) (Lepidoptera: Noctuidae)". Animal Behaviour. 18, Part 2: 310–316. doi:10.1016/S0003-3472(70)80043-4.
- ↑ Heath, R. R.; Mclaughlin, J. R.; Proshold, F.; Teal, P. E. A. (1 March 1991). "Periodicity of Female Sex Pheromone Titer and Release in Heliothis subflexa and H. virescens (Lepidoptera: Noctuidae)". Annals of the Entomological Society of America. 84 (2): 182–189. doi:10.1093/aesa/84.2.182. ISSN 0013-8746.
- ↑ Ronkay, L. (2005). "Revision of the genus Lophoterges Hampson, 1906 (s. l.) (Lepidoptera, Noctuidae, Cuculliinae). Part II. The genus Lophoterges s. str". Acta Zoologica Academiae Scientiarum Hungaricae. 51: 1–57.
- 1 2 3 4 Wagner, David L.; Schweitzer, Dale F.; Sullivan, J. Bolling & Reardon, Richard C. (2011). Owlet Caterpillars of Eastern North America. Princeton University Press. ISBN 978-0691150420.
- ↑ Vilanova, Cristina; Baixeras, Joaquín; Latorre, Amparo; Porcar, Manuel (1 January 2016). "The Generalist Inside the Specialist: Gut Bacterial Communities of Two Insect Species Feeding on Toxic Plants Are Dominated by Enterococcus sp". Frontiers in Microbiology. 7: 1005. doi:10.3389/fmicb.2016.01005. ISSN 1664-302X. PMC 4923067. PMID 27446044.
- ↑ Nakamura, M. (1998). "The eversible cervical gland and the chemical component of its secretion in noctuid larvae" (PDF). Transactions of the Lepidopterological Society of Japan. 49: 85–92.
- ↑ Smedley, Scott R.; Ehrhardt, Elizabeth; Eisner, Thomas (1993). "Defensive Regurgitation by a Noctuid Moth Larva (Litoprosopus futilis)". Psyche: A Journal of Entomology. 100 (3–4): 209–221. doi:10.1155/1993/67950. ISSN 0033-2615.
- ↑ Kristensen, Niels (1 January 2003). Handbook of Zoology. Vol. 2: Morphology, Physiology, and Development. Walter de Gruyter. ISBN 9783110893724.
- ↑ Narayanamma, V. Lakshmi; Sharma, H. C.; Gowda, C. L. L.; Sriramulu, M. (1 December 2007). "Incorporation of lyophilized leaves and pods into artificial diets to assess the antibiosis component of resistance to pod borer Helicoverpa armigera (Lepidoptera: Noctuidae) in chickpea" (PDF). International Journal of Tropical Insect Science. 27 (3–4): 191–198. doi:10.1017/S1742758407878374. ISSN 1742-7592. S2CID 86757646.
- ↑ Capinera, John L. (2008). "Variegated Cutworm, Peridroma saucia (Hübner) (Lepidoptera: Noctuidae)". In Capinera, John L. (ed.). Encyclopedia of Entomology. Springer Netherlands. pp. 4038–4041. doi:10.1007/978-1-4020-6359-6_3936. ISBN 9781402062421.
- ↑ Heath, Jeffrey. "Guide to insects, arthropods, and molluscs of northern Dogon country".
- ↑ Keegan, Kevin; Rota, Jadranka; Zahiri, Reza; Zilli, Alberto; et al. (2021). "Toward a Stable Global Noctuidae (Lepidoptera) Taxonomy". Insect Systematics and Diversity. 5 (3). doi:10.1093/isd/ixab005.
- ↑ Wagner, David L.; Keegan, Kevin; Bugh, Valerie G. (2019). "A Tale of Two Caterpillars and Reclassification of Cerathosia Smith and Cydosia Duncan [& Westwood] (Lepidoptera: Noctuidae)". Journal of the Lepidopterists' Society. 73 (1): 1. doi:10.18473/lepi.73i1.a1.
External links
- On University of Florida Institute of Food and Agricultural Sciences Featured Creatures web site:
- Agrotis ipsilon, black cutworm
- Diphthera festiva, hieroglyphic moth
- Litoprosopus futilis , cabbage palm caterpillar
- Pseudaletia unipuncta, true armyworm
- Spodoptera eridania, southern armyworm
- Spodoptera frugiperda, fall armyworm
- Spodoptera ornithogalli, yellowstriped armyworm
- Xanthopastis timais, Spanish moth or convict caterpillar
- Images of Noctuidae species in New Zealand