In biochemistry, non-heme iron proteins describe families of enzymes that utilize iron at the active site but lack heme cofactors. Iron-sulfur proteins, including those that are enzymes, are not included in this definition.
Some non-heme iron proteins contain one Fe at their active sites, others have pairs of Fe centers:
- Many mono-Fe proteins are alpha-ketoglutarate-dependent hydroxylases. Major examples are the lipoxygenases, isopenicillin N synthase, protocatechuate 3,4-dioxygenase, deacetoxycephalosporin-C synthase,[1] and aromatic amino acid hydroxylases.[2]
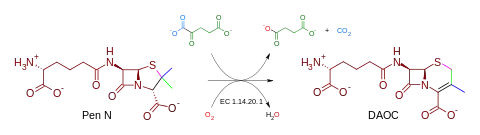
Illustrative transformation catalyzed by a non-heme iron protein.
- Major diiron enzymes include hemerythrin, some ribonucleotide reductases,[3] some methane monooxygenases, purple acid phosphatases, and ferritin.[4][5]
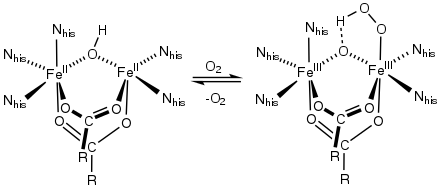 Oxygenation of hemerythrin, a non-heme diiron protein.
Oxygenation of hemerythrin, a non-heme diiron protein.
See also
References
- ↑ Que, Lawrence; Ho, Raymond Y. N. (1996). "Dioxygen Activation by Enzymes with Mononuclear Non-Heme Iron Active Sites". Chemical Reviews. 96 (7): 2607–2624. doi:10.1021/cr960039f. PMID 11848838.
- ↑ Abu-Omar, Mahdi M.; Loaiza, Aristobulo; Hontzeas, Nikos (2005). "Reaction Mechanisms of Mononuclear Non-Heme Iron Oxygenases". Chemical Reviews. 105 (6): 2227–2252. doi:10.1021/cr040653o. PMID 15941213.
- ↑ Stubbe, Joanne; Nocera, Daniel G.; Yee, Cyril S.; Chang, Michelle C. Y. (2003). "Radical Initiation in the Class I Ribonucleotide Reductase: Long-Range Proton-Coupled Electron Transfer?". Chemical Reviews. 103 (6): 2167–2202. doi:10.1021/cr020421u. PMID 12797828.
- ↑ Tshuva, Edit Y.; Lippard, Stephen J. (2004). "Synthetic Models for Non-Heme Carboxylate-Bridged Diiron Metalloproteins: Strategies and Tactics". Chemical Reviews. 104 (2): 987–1012. doi:10.1021/cr020622y. PMID 14871147.
- ↑ Wallar, Bradley J.; Lipscomb, John D. (1996). "Dioxygen Activation by Enzymes Containing Binuclear Non-Heme Iron Clusters". Chemical Reviews. 96 (7): 2625–2658. doi:10.1021/cr9500489. PMID 11848839.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.