 | |
 | |
| Names | |
|---|---|
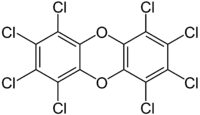
| Preferred IUPAC name
Octachlorooxanthrene | |
| Other names
OCDD, Octachlorodibenzo-p-dioxin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.223.031 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C12Cl8O2 | |
| Molar mass | 459.75 g/mol |
| Related compounds | |
Related Polychlorinated dibenzodioxins |
Tetrachlorodibenzodioxin |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Octachlorodibenzodioxin (can be abbreviated as OCDD or OctaCDD) is one of polychlorinated dibenzodioxins (PCDDs).
Its toxicity is similar to TCDD, but it is about 3000 times weaker.[1]
It is one of the dominant dioxin congeners in smoke emissions, but because it is much less soluble than e.g. TCDD, it is not bioaccumulated as effectively and it is toxicologically less important than the most prevalent dioxins.
(for details see Dioxins and dioxin-like compounds).
Chlorinated pesticides can also contain impurities of dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and their precursors. "Precursor formation of PCDD/Fs can also be mediated by ultraviolet light (UV)" from sun (OCDD mostly). Changes in congeners ratio after UV exposition suggest that pesticide sources of dioxines-like after sunlight exposure "may not be recognized based on matching source fingerprints established from manufacturing impurities. These changes also provide preliminary insights into the possible formation routes and types of precursors involved".[2]
References
- ↑ M. Van den Berg, L.S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, M. Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker, R.E. Peterson, The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds, Toxicol. Sci. 93 (2006) 223–241.
- ↑ Holt, E., Weber, R., Stevenson, G., & Gaus, C. (2012) Formation of dioxins during exposure of pesticide formulations to sunlight. Chemosphere, 88(3), 364-370.