 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Heptafluorobutanoic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | HFBA |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.006.170 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4HF7O2 | |
| Molar mass | 214.039 g·mol−1 |
| Appearance | colourless liquid |
| Density | 1.64 g/ml |
| Boiling point | 120 °C (248 °F; 393 K) |
| high | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
strong acid |
| GHS labelling:[2] | |
 | |
| Danger | |
| H314 | |
| P260, P280, P303+P361+P353, P304+P340+P310, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
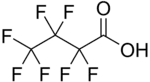
Perfluorobutanoic acid (PFBA) is a perfluoroalkyl carboxylic acid with the formula C3F7CO2H. As the perfluorinated derivative of butyric acid, this colourless liquid is prepared by electrofluorination of the corresponding butyryl fluoride.[3]
Applications
PFBA has a variety of niche applications in analytical and synthetic chemistry. It is an ion pair reagent for reverse-phase HPLC. It is used in the sequencing, synthesis, and solubilizing of proteins and peptides.
Esters derived from PFBA readily undergo condensation, owing to their electrophilicity. Specialized ligands for metal ions are generated capitalizing on this property, such as Eufod.
PFBA was formerly used for manufacturing photographic film. The 3M Company was a major manufacturer of PFBA and products containing PFBA but production was phased out in 1998.[4]
Environmental impact and regulation
PFBA is a breakdown product of other PFAS that have been used in stain-resistant fabrics, paper food packaging, carpets, and consumer products.[5] PFBA has been frequently found in U.S. rivers.[4][6]
In laboratory animal studies, exposure to high levels of PFBA results in thyroid and liver effects, such as increased thyroid and liver weight, changes in thyroid hormones, decreased cholesterol, and cellular changes in both organs. Other effects of PFBA exposure included delayed development and decreased red blood cells and hemoglobin.[4]
The Minnesota Department of Health (MDH) developed a guidance value of 7 ppb for PFBA in drinking water.[4]
References
- ↑ Buck, Robert C; Franklin, James; Berger, Urs; Conder, Jason M; Cousins, Ian T; de Voogt, Pim; Jensen, Allan Astrup; Kannan, Kurunthachalam; Mabury, Scott A; van Leeuwen, Stefan PJ (2011). "Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins". Integrated Environmental Assessment and Management. 7 (4): 513–541. doi:10.1002/ieam.258. PMC 3214619. PMID 21793199.
- ↑ Sigma-Aldrich Co., Heptafluorobutyric acid. Retrieved on 2022-03-23.
- ↑ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000), "Fluorine Compounds, Organic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, doi:10.1002/14356007.a11_349, ISBN 978-3-527-30673-2
- 1 2 3 4 "Perfluorobutanoic acid (PFBA) and Water" (PDF). Minnesota Department of Health. April 2022. Retrieved 8 September 2023.
- ↑ "EPA Publishes IRIS Handbook and Final IRIS Assessment of Perfluorobutanoic Acid (PFBA) and Related Salts". US EPA. December 22, 2022. Retrieved 8 September 2023.
- ↑ Chase, Richard (2020). "Results of Per- and Polyfluoroalkyl Substances (PFAS) in Selected Brooks and Rivers in Massachusetts, 2020". Massachusetts Department of Environmental Protection. Retrieved 8 September 2023.