

In the field of food processing, pasteurization (also pasteurisation) is a process of food preservation in which packaged and unpacked foods (e.g., milk and fruit juices) are treated with mild heat, usually to less than 100 °C (212 °F), to eliminate pathogens and extend shelf life. Pasteurization either destroys or deactivates microorganisms and enzymes that contribute to food spoilage or the risk of disease, including vegetative bacteria, but most bacterial spores survive the process.[1][2]
The process of pasteurization is named after the French microbiologist Louis Pasteur, whose research in the 1860s demonstrated that thermal processing would deactivate unwanted microorganisms in wine.[2][3] Spoilage enzymes are also inactivated during pasteurization. Today, pasteurization is used widely in the dairy industry and other food processing industries to achieve food preservation and food safety.[3]
By the year 1999, most liquid products were heat treated in a continuous system where heat can be applied using a heat exchanger or the direct or indirect use of hot water and steam. Due to the mild heat, there are minor changes to the nutritional quality and sensory characteristics of the treated foods.[4] Pascalization or high pressure processing (HPP) and pulsed electric field (PEF) are non-thermal processes that are also used to pasteurize foods.[1]
History

The process of heating wine for preservation purposes has been known in China since AD 1117, and was documented in Japan in the diary Tamonin-nikki, written by a series of monks between 1478 and 1618.[5]
Much later, in 1768, research performed by Italian priest and scientist Lazzaro Spallanzani proved a product could be made "sterile" after thermal processing. Spallanzani boiled meat broth for one hour, sealed the container immediately after boiling, and noticed that the broth did not spoil and was free from microorganisms.[2][6] In 1795, a Parisian chef and confectioner named Nicolas Appert began experimenting with ways to preserve foodstuffs, succeeding with soups, vegetables, juices, dairy products, jellies, jams, and syrups. He placed the food in glass jars, sealed them with cork and sealing wax and placed them in boiling water.[7] In that same year, the French military offered a cash prize of 12,000 francs for a new method to preserve food. After some 14 or 15 years of experimenting, Appert submitted his invention and won the prize in January 1810.[8] Later that year, Appert published L'Art de conserver les substances animales et végétales ("The Art of Preserving Animal and Vegetable Substances"). This was the first cookbook of its kind on modern food preservation methods.[9][10]
La Maison Appert (English: The House of Appert), in the town of Massy, near Paris, became the first food-bottling factory in the world,[7] preserving a variety of foods in sealed bottles. Appert's method was to fill thick, large-mouthed glass bottles with produce of every description, ranging from beef and fowl to eggs, milk and prepared dishes. He left air space at the top of the bottle, and the cork would then be sealed firmly in the jar by using a vise. The bottle was then wrapped in canvas to protect it while it was dunked into boiling water and then boiled for as much time as Appert deemed appropriate for cooking the contents thoroughly. Appert patented his method, sometimes called appertisation in his honor.[11]
Appert's method was so simple and workable that it quickly became widespread. In 1810, British inventor and merchant Peter Durand, also of French origin, patented his own method, but this time in a tin can, so creating the modern-day process of canning foods. In 1812, Englishmen Bryan Donkin and John Hall purchased both patents and began producing preserves. Just a decade later, Appert's method of canning had made its way to America.[12] Tin can production was not common until the beginning of the 20th century, partly because a hammer and chisel were needed to open cans until the invention of a can opener by Robert Yeates in 1855.[7]
A less aggressive method was developed by French chemist Louis Pasteur during an 1864[13] summer holiday in Arbois. To remedy the frequent acidity of the local aged wines, he found out experimentally that it is sufficient to heat a young wine to only about 50–60 °C (122–140 °F) for a short time to kill the microbes, and that the wine could subsequently be aged without sacrificing the final quality.[13] In honour of Pasteur, this process is known as "pasteurization".[2][14] Pasteurization was originally used as a way of preventing wine and beer from souring,[15] and it would be many years before milk was pasteurized. In the United States in the 1870s, before milk was regulated, it was common for milk to contain substances intended to mask spoilage.[16]
Milk

Milk is an excellent medium for microbial growth,[17] and when it is stored at ambient temperature bacteria and other pathogens soon proliferate.[18] The US Centers for Disease Control (CDC) says improperly handled raw milk is responsible for nearly three times more hospitalizations than any other food-borne disease source, making it one of the world's most dangerous food products.[19][20] Diseases prevented by pasteurization can include tuberculosis, brucellosis, diphtheria, scarlet fever, and Q-fever; it also kills the harmful bacteria Salmonella, Listeria, Yersinia, Campylobacter, Staphylococcus aureus, and Escherichia coli O157:H7,[21][22] among others.
Prior to industrialization, dairy cows were kept in urban areas to limit the time between milk production and consumption, hence the risk of disease transmission via raw milk was reduced.[23] As urban densities increased and supply chains lengthened to the distance from country to city, raw milk (often days old) became recognized as a source of disease. For example, between 1912 and 1937, some 65,000 people died of tuberculosis contracted from consuming milk in England and Wales alone.[24] Because tuberculosis has a long incubation period in humans, it was difficult to link unpasteurized milk consumption with the disease.[25] In 1892, chemist Ernst Lederle experimentally inoculated milk from tuberculosis-diseased cows into guinea pigs, which caused them to develop the disease.[26] In 1910, Lederle, then in the role of Commissioner of Health, introduced mandatory pasteurization of milk in New York City.[26]
Developed countries adopted milk pasteurization to prevent such disease and loss of life, and as a result milk is now considered a safer food.[27] A traditional form of pasteurization by scalding and straining of cream to increase the keeping qualities of butter was practiced in Great Britain in the 18th century and was introduced to Boston in the British Colonies by 1773,[28] although it was not widely practiced in the United States for the next 20 years. Pasteurization of milk was suggested by Franz von Soxhlet in 1886.[29] In the early 20th century, Milton Joseph Rosenau established the standards – i.e. low-temperature, slow heating at 60 °C (140 °F) for 20 minutes – for the pasteurization of milk[30][31] while at the United States Marine Hospital Service, notably in his publication of The Milk Question (1912).[32] States in the U.S. soon began enacting mandatory dairy pasteurization laws, with the first in 1947, and in 1973 the U.S. federal government required pasteurization of milk used in any interstate commerce.[33]
The shelf life of refrigerated pasteurized milk is greater than that of raw milk. For example, high-temperature, short-time (HTST) pasteurized milk typically has a refrigerated shelf life of two to three weeks, whereas ultra-pasteurized milk can last much longer, sometimes two to three months. When ultra-heat treatment (UHT) is combined with sterile handling and container technology (such as aseptic packaging), it can even be stored non-refrigerated for up to 9 months.[34]
According to the Centers for Disease Control, between 1998 and 2011, 79% of dairy-related disease outbreaks in the United States were due to raw milk or cheese products.[35] They report 148 outbreaks and 2,384 illnesses (with 284 requiring hospitalization), as well as two deaths due to raw milk or cheese products during the same time period.[35]
Medical equipment
Medical equipment, notably respiratory and anesthesia equipment, is often disinfected using hot water, as an alternative to chemical disinfection. The temperature is raised to 70 °C (158 °F) for 30 minutes.[36]
Pasteurization process
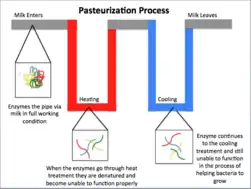
Pasteurization is a mild heat treatment of liquid foods (both packaged and unpackaged) where products are typically heated to below 100 °C. The heat treatment and cooling process are designed to inhibit a phase change of the product. The acidity of the food determines the parameters (time and temperature) of the heat treatment as well as the duration of shelf life. Parameters also take into account nutritional and sensory qualities that are sensitive to heat.
In acidic foods (pH <4.6), such as fruit juice and beer, the heat treatments are designed to inactivate enzymes (pectin methylesterase and polygalacturonase in fruit juices) and destroy spoilage microbes (yeast and lactobacillus). Due to the low pH of acidic foods, pathogens are unable to grow. The shelf-life is thereby extended several weeks. In less acidic foods (pH >4.6), such as milk and liquid eggs, the heat treatments are designed to destroy pathogens and spoilage organisms (yeast and molds). Not all spoilage organisms are destroyed under pasteurization parameters, so subsequent refrigeration is necessary.[1]
High-temperature short-time (HTST) pasteurization, such as that used for milk (71.5 °C (160.7 °F) for 15 seconds) ensures safety of milk and provides a refrigerated shelf life of approximately two weeks. In ultra-high-temperature (UHT) pasteurization, milk is pasteurized at 135 °C (275 °F) for 1–2 seconds, which provides the same level of safety, but along with the packaging, extends shelf life to three months under refrigeration.[37]
Equipment
Food can be pasteurized either before or after being packaged into containers. Pasteurization of food in containers generally uses either steam or hot water. When food is packaged in glass, hot water is used to avoid cracking the glass from thermal shock. When plastic or metal packaging is used, the risk of thermal shock is low, so steam or hot water is used.[1]
Most liquid foods are pasteurized by using a continuous process that passes the food through a heating zone, a hold tube to keep it at the pasteurization temperature for the desired time, and a cooling zone, after which the product is filled into the package. Plate heat exchangers are often used for low-viscosity products such as animal milks, nut milks and juices. A plate heat exchanger is composed of many thin vertical stainless steel plates that separate the liquid from the heating or cooling medium.
Shell and tube heat exchangers are often used for the pasteurization of foods that are non-Newtonian fluids, such as dairy products, tomato ketchup and baby foods. A tube heat exchanger is made up of concentric stainless steel tubes. Food passes through the inner tube or tubes, while the heating/cooling medium is circulated through the outer tube.
Scraped-surface heat exchangers are a type of shell and tube which contain an inner rotating shaft having spring-loaded blades that serve to scrape away any highly viscous material that accumulates on the wall of the tube.[38]
The benefits of using a heat exchanger to pasteurize foods before packaging, versus pasteurizing foods in containers are:
- Higher uniformity of treatment
- Greater flexibility with regard to the products that can be pasteurized
- Higher heat transfer-efficiency[1]
- Greater throughput
After being heated in a heat exchanger, the product flows through a hold tube for a set period of time to achieve the required treatment. If pasteurization temperature or time is not achieved, a flow diversion valve is used to divert under-processed product back to the raw product tank.[39] If the product is adequately processed, it is cooled in a heat exchanger, then filled.
Verification
Direct microbiological techniques are the ultimate measurement of pathogen contamination, but these are costly and time-consuming, which means that products have a reduced shelf-life by the time pasteurization is verified.
As a result of the unsuitability of microbiological techniques, milk pasteurization efficacy is typically monitored by checking for the presence of alkaline phosphatase, which is denatured by pasteurization. Destruction of alkaline phosphatase ensures the destruction of common milk pathogens. Therefore, the presence of alkaline phosphatase is an ideal indicator of pasteurization efficacy.[40][41] For liquid eggs, the effectiveness of the heat treatment is measured by the residual activity of α-amylase.[1]
Efficacy against pathogenic bacteria
During the early 20th century, there was no robust knowledge of what time and temperature combinations would inactivate pathogenic bacteria in milk, and so a number of different pasteurization standards were in use. By 1943, both HTST pasteurization conditions of 72 °C (162 °F) for 15 seconds, as well as batch pasteurization conditions of 63 °C (145 °F) for 30 minutes, were confirmed by studies of the complete thermal death (as best as could be measured at that time) for a range of pathogenic bacteria in milk.[42] Complete inactivation of Coxiella burnetii (which was thought at the time to cause Q fever by oral ingestion of infected milk)[43][44] as well as of Mycobacterium tuberculosis (which causes tuberculosis)[45] were later demonstrated. For all practical purposes, these conditions were adequate for destroying almost all yeasts, molds, and common spoilage bacteria and also for ensuring adequate destruction of common pathogenic, heat-resistant organisms. However, the microbiological techniques used until the 1960s did not allow for the actual reduction of bacteria to be enumerated. Demonstration of the extent of inactivation of pathogenic bacteria by milk pasteurization came from a study of surviving bacteria in milk that was heat-treated after being deliberately spiked with high levels of the most heat-resistant strains of the most significant milk-borne pathogens.[46]
The mean log10 reductions and temperatures of inactivation of the major milk-borne pathogens during a 15-second treatment are:
- Staphylococcus aureus > 6.7 at 66.5 °C (151.7 °F)
- Yersinia enterocolitica > 6.8 at 62.5 °C (144.5 °F)
- Pathogenic Escherichia coli > 6.8 at 65 °C (149 °F)
- Cronobacter sakazakii > 6.7 at 67.5 °C (153.5 °F)
- Listeria monocytogenes > 6.9 at 65.5 °C (149.9 °F)
- Salmonella ser. Typhimurium > 6.9 at 61.5 °C (142.7 °F)[46]
(A log10 reduction between 6 and 7 means that 1 bacterium out of 1 million (106) to 10 million (107) bacteria survive the treatment.)
The Codex Alimentarius Code of Hygienic Practice for Milk notes that milk pasteurization is designed to achieve at least a 5 log10 reduction of Coxiella burnetii.[47] The Code also notes that: "The minimum pasteurization conditions are those having bactericidal effects equivalent to heating every particle of the milk to 72 °C for 15 seconds (continuous flow pasteurization) or 63 °C for 30 minutes (batch pasteurization)” and that "To ensure that each particle is sufficiently heated, the milk flow in heat exchangers should be turbulent, i.e. the Reynolds number should be sufficiently high". The point about turbulent flow is important because simplistic laboratory studies of heat inactivation that use test tubes, without flow, will have less bacterial inactivation than larger-scale experiments that seek to replicate conditions of commercial pasteurization.[48]
As a precaution, modern HTST pasteurization processes must be designed with flow-rate restriction as well as divert valves which ensure that the milk is heated evenly and that no part of the milk is subject to a shorter time or a lower temperature. It is common for the temperatures to exceed 72 °C by 1.5 °C or 2 °C.[48]
Double pasteurization
Pasteurization is not sterilization and does not kill spores. "Double" pasteurization, which involves a secondary heating process, can extend shelf life by killing spores that have germinated.[49]
The acceptance of double pasteurization varies by jurisdiction. In places where it is allowed, milk is initially pasteurized when it is collected from the farm so it does not spoil before processing. Many countries prohibit the labelling of such milk as "pasteurized" but allow it to be marked "thermized", which refers to a lower-temperature process.[50]
Effects on nutritional and sensory characteristics of foods
Because of its mild heat treatment, pasteurization increases the shelf-life by a few days or weeks.[1] However, this mild heat also means there are only minor changes to heat-labile vitamins in the foods.[4]
Milk
According to a systematic review and meta-analysis,[51] it was found that pasteurization appeared to reduce concentrations of vitamins B12 and E, but it also increased concentrations of vitamin A. However, in the review, there was only limited research regarding how much pasteurization affects A, B12, and E levels.[51] Milk is not considered an important source of vitamins B12 or E in the North American diet, so the effects of pasteurization on the adult daily intake of these vitamins is negligible.[52][53] However, milk is considered an important source of vitamin A,[54] and because pasteurization appears to increase vitamin A concentrations in milk, the effect of milk heat treatment on this vitamin is a not a major public health concern.[51] Results of meta-analyses reveal that pasteurization of milk leads to a significant decrease in vitamin C and folate, but milk is also not an important source of these vitamins.[54][53] A significant decrease in vitamin B2 concentrations was found after pasteurization. Vitamin B2 is typically found in bovine milk at concentrations of 1.83 mg/liter. Because the recommended daily intake for adults is 1.1 mg/day,[52] milk consumption greatly contributes to the recommended daily intake of this vitamin. With the exception of B2, pasteurization does not appear to be a concern in diminishing the nutritive value of milk because milk is often not a primary source of these studied vitamins in the North American diet.
Sensory effects
Pasteurization also has a small but measurable effect on the sensory attributes of the foods that are processed.[1] In fruit juices, pasteurization may result in loss of volatile aroma compounds.[4] Fruit juice products undergo a deaeration process prior to pasteurization that may be responsible for this loss. Deaeration also minimizes the loss of nutrients like vitamin C and carotene.[1] To prevent the decrease in quality resulting from the loss in volatile compounds, volatile recovery, though costly, can be utilized to produce higher-quality juice products.[4]
In regard to color, the pasteurization process does not have much effect on pigments such as chlorophylls, anthocyanins, and carotenoids in plants and animal tissues. In fruit juices, polyphenol oxidase (PPO) is the main enzyme responsible for causing browning and color changes. However, this enzyme is deactivated in the deaeration step prior to pasteurization with the removal of oxygen.[4]
In milk, the color difference between pasteurized and raw milk is related to the homogenization step that takes place prior to pasteurization. Before pasteurization milk is homogenized to emulsify its fat and water-soluble components, which results in the pasteurized milk having a whiter appearance compared to raw milk.[1] For vegetable products, color degradation is dependent on the temperature conditions and the duration of heating.[55]
Pasteurization may result in some textural loss as a result of enzymatic and non-enzymatic transformations in the structure of pectin if the processing temperatures are too high as a result. However, with mild heat treatment pasteurization, tissue softening in the vegetables that causes textural loss is not of concern as long as the temperature does not get above 80 °C (176 °F).[55]
Novel pasteurization methods
"Pasteurizing" in the broad sense refers to any method that reduces microbes by an amount (log reduction) equivalent to Pasteur's process. Novel processes, thermal and non-thermal, have been developed to pasteurize foods as a way of reducing the effects on nutritional and sensory characteristics of foods and preventing degradation of heat-labile nutrients. Pascalization or high pressure processing (HPP),[1][56][57] pulsed electric field (PEF),[1][56][57] ionising radiation, high pressure homogenisation, UV decontamination, pulsed high intensity light, high intensity laser, pulsed white light, high power ultrasound, oscillating magnetic fields, high voltage arc discharge, and streamer plasma[56][57] are examples of these non-thermal pasteurization methods that are currently commercially utilized.
Microwave volumetric heating (MVH) is the newest available pasteurization technology. It uses microwaves to heat liquids, suspensions, or semi-solids in a continuous flow. Because MVH delivers energy evenly and deeply into the whole body of a flowing product, it allows for gentler and shorter heating, so that almost all heat-sensitive substances in the milk are preserved.[58]
Products that are commonly pasteurized
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 Fellows, P. J. (2017). Food Processing Technology Principles and Practice. Woodhead Publishing Series in Food Science, Technology and Nutrition. pp. 563–578. ISBN 978-0-08-101907-8.
- 1 2 3 4 Tewari, Gaurav; Juneja, Vijay K. (2007). Advances in Thermal and Non-Thermal Food Preservation. Blackwell Publishing. pp. 3, 96, 116. ISBN 9780813829685.
- 1 2 "Heat Treatments and Pasteurisation". milkfacts.info. Archived from the original on 5 June 2007. Retrieved 12 December 2016.
- 1 2 3 4 5 Rahman, M. Shafiur (21 January 1999). Handbook of Food Preservation. CRC Press. ISBN 978-0-8247-0209-0. Archived from the original on 19 July 2022. Retrieved 30 March 2021.
- ↑ Hornsey, Ian Spencer and George Bacon (2003). A History of Beer and Brewing. Royal Society of Chemistry. p. 30. ISBN 978-0-85404-630-0. Archived from the original on 12 August 2020. Retrieved 2 January 2011.
[…] sake is pasteurized and it is interesting to note that a pasteurization technique was first mentioned in 1568 in the _Tamonin-nikki_, the diary of a Buddhist monk, indicating that it was practiced in Japan some 300 years before Pasteur. In China, the first country in East Asia to develop a form of pasteurization, the earliest record of the process is said to date from 1117.
- ↑ Vallery-Radot, René (1 March 2003). Life of Pasteur 1928. Kessinger. pp. 113–14. ISBN 978-0-7661-4352-4. Archived from the original on 1 January 2016. Retrieved 8 January 2016.
- 1 2 3 Lance Day, Ian McNeil, ed. (1996). Biographical Dictionary of the History of Technology. Routledge. ISBN 978-0-415-19399-3.
- ↑ Gordon L. Robertson (1998). Food Packaging: Principles End Practice. Marcel Dekker. p. 187. ISBN 978-0-8247-0175-8. Archived from the original on 19 August 2020. Retrieved 8 January 2016.
- ↑ "The First Book on Modern Food Preservation Methods (1810)". Historyofscience.com. 29 September 2009. Archived from the original on 1 January 2011. Retrieved 19 March 2014.
- ↑ Wiley, R. C (1994). Minimally processed refrigerated fruits and vegetables. Springer. p. 66. ISBN 978-0-412-05571-3. Archived from the original on 19 August 2020. Retrieved 8 January 2016.
Nicolas Appert in 1810 was probably the first person […]
- ↑ Garcia, Adrian, Rebeca, Jean (March 2009). "Nicolas Appert: Inventor and Manufacturer". Food Reviews International. 25 (2): 115–125. doi:10.1080/87559120802682656. S2CID 83865891.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Alvin Toffler, "Future Shock".
- 1 2 Vallery-Radot, René (1 March 2003). Life of Pasteur 1928. pp. 113–14. ISBN 978-0-7661-4352-4. Archived from the original on 19 July 2022. Retrieved 22 December 2021.
- ↑ "History – Louis Pasteur". BBC. Archived from the original on 3 May 2015. Retrieved 25 December 2019.
- ↑ Carlisle, Rodney (2004). Scientific American Inventions and Discoveries, p. 357. John Wiley & Songs, Inc., new Jersey. ISBN 0-471-24410-4.
- ↑ Hwang, Andy; Huang, Lihan (31 January 2009). Ready-to-Eat Foods: Microbial Concerns and Control Measures. CRC Press. p. 88. ISBN 978-1-4200-6862-7. Archived from the original on 2 June 2013. Retrieved 19 April 2011.
- ↑ "Harold Eddleman, Making Milk Media, Indiana Biolab". Disknet.com. Archived from the original on 13 May 2013. Retrieved 19 March 2014.
- ↑ "Frank O'Mahony, Rural dairy technology: Experiences in Ethiopia, International Livestock Centre for Africa". Ilri.org. Archived from the original on 20 February 2014. Retrieved 19 March 2014.
- ↑ "Food safety of raw milk". Foodsmart.govt.nz. Archived from the original on 8 April 2014. Retrieved 19 March 2014.
- ↑ Langer, Adam J.; Ayers, Tracy; Grass, Julian; Lynch, Michael; Angulo, Frederick; Mahon, Barbara (2012). "Nonpasteurized Dairy Products, Disease Outbreaks, and State Laws – United States, 1993–2006" (PDF). Emerging Infectious Diseases. 18 (3): 385–91. doi:10.3201/eid1803.111370. PMC 3309640. PMID 22377202. Archived from the original (PDF) on 23 August 2015. Retrieved 11 February 2015.
- ↑ "Milk Pasteurization: Guarding against disease", Michigan State University Extension
- ↑ Smith, P.W., (August 1981), "Milk Pasteurization" Fact Sheet Number 57, U.S. Department of Agriculture Research Service, Washington, DC
- ↑ ABB, Inc. (2018), Recording and Control C1900 in Pasteurization processes (PDF), archived (PDF) from the original on 17 May 2018, retrieved 17 May 2018
{{citation}}:|first=has generic name (help) - ↑ Wilson, G.S. (1943), "The Pasteurization of Milk", British Medical Journal, 1 (4286): 261–62, doi:10.1136/bmj.1.4286.261, PMC 2282302, PMID 20784713
- ↑ Pearce, Lindsay (2002). "Bacterial diseases – The impact of milk processing to reduce risks". Bulletin of the International Dairy Federation. 372: 20–25. ISSN 0250-5118. Archived from the original on 29 July 2017. Retrieved 23 June 2017.
- 1 2 Weinstein, I (1947). "Eighty years of public health in New York City". Bulletin of the New York Academy of Medicine. 23 (4): 221–237. PMC 1871552. PMID 19312527.
- ↑ ABB, Inc. (2018), Recording and Control C1900 in Pasteurization processes (PDF), archived (PDF) from the original on 17 May 2018, retrieved 17 May 2018
{{citation}}:|first=has generic name (help) - ↑ Kaden H. 2017. Food Preservation tools and techniques: In Food Industry process and technologies. Library press. pages 129–178
- ↑ Franz Soxhlet (1886) "Über Kindermilch und Säuglings-Ernährung" (On milk for babies and infant nutrition), Münchener medizinische Wochenschrift (Munich Medical Weekly), vol. 33, pp. 253, 276.
- ↑ "January 1: Pasteurization". Jewish Currents. 1 January 2015. Archived from the original on 4 January 2015. Retrieved 4 January 2015.
- ↑ "Milton J. Rosenau, M.D." www.cdc.gov. Archived from the original on 23 August 2013. Retrieved 7 September 2017.
- ↑ Details – The milk question. Houghton Mifflin company. 1912. Archived from the original on 2 June 2018. Retrieved 14 January 2018.
{{cite book}}:|website=ignored (help) - ↑ "Federal and State Regulation of Raw Milk" (PDF). Archived (PDF) from the original on 6 February 2017. Retrieved 23 July 2016.
- ↑ ABB, Inc. (2018), Recording and Control C1900 in Pasteurization processes (PDF), archived (PDF) from the original on 17 May 2018, retrieved 17 May 2018
{{citation}}:|first=has generic name (help) - 1 2 "Raw Milk Questions and Answers – Food Safety". Centers for Disease Control. 7 March 2014. Archived from the original on 30 July 2017. Retrieved 19 March 2014.
- ↑ "Guideline for Disinfection and Sterilization in Healthcare Facilities". Centers for Disease Control. 2008. Archived from the original on 11 July 2019. Retrieved 10 July 2018.
- ↑ Chavan, Rupesh S.; Chavan, Shraddha Rupesh; Khedkar, Chandrashekar D.; Jana, Atanu H. (22 August 2011). "UHT Milk Processing and Effect of Plasmin Activity on Shelf Life: A Review". Comprehensive Reviews in Food Science and Food Safety. 10 (5): 251–68. doi:10.1111/j.1541-4337.2011.00157.x. ISSN 1541-4337.
- ↑ Smith, P. G (2003). Introduction to Food Process Engineering. Food Science Text Series. pp. 152–54, 259–50.
- ↑ (Kosebalaban) Tokatli, Figen; Cinar, Ali; Schlesser, Joseph E. (1 June 2005). "HACCP with multivariate process monitoring and fault diagnosis techniques: application to a food pasteurization process". Food Control. 16 (5): 411–422. doi:10.1016/j.foodcont.2004.04.008. hdl:11147/1960. ISSN 0956-7135.
- ↑ Kay, H. (1935). "Some Results of the Application of a Simple Test for Efficiency of Pasteurization". The Lancet. 225 (5835): 1516–18. doi:10.1016/S0140-6736(01)12532-8.
- ↑ Hoy, W.A.; Neave, F.K. (1937). "The Phosphatase Test for Efficient Pasteurization". The Lancet. 230 (5949): 595. doi:10.1016/S0140-6736(00)83378-4.
- ↑ Ball, C. Olin (1 January 1943). "Short-Time Pasteurization of Milk". Industrial & Engineering Chemistry. 35 (1): 71–84. doi:10.1021/ie50397a017. ISSN 0019-7866.
- ↑ Enright, J.B.; Sadler, W.W.; Thomas, R.C. (1957). "Thermal inactivation of Coxiella burnetii and its relation to pasteurization of milk". Public Health Monograph. 47: 1–30. ISSN 0079-7596. PMID 13465932.
- ↑ Cerf, O.; Condron, R. (2006). "Coxiella burnetii and milk pasteurization: an early application of the precautionary principle?". Epidemiology & Infection. 134 (5): 946–51. doi:10.1017/S0950268806005978. ISSN 1469-4409. PMC 2870484. PMID 16492321.
- ↑ Kells, H.R.; Lear, S.A. (1 July 1960). "Thermal Death Time Curve of Mycobacterium tuberculosis var. bovis in Artificially Infected Milk". Applied Microbiology. 8 (4): 234–236. doi:10.1128/am.8.4.234-236.1960. ISSN 0099-2240. PMC 1057612. PMID 14405283.
- 1 2 Pearce, L.E.; Smythe, B.W.; Crawford, R.A.; Oakley, E.; Hathaway, S.C.; Shepherd, J.M. (2012). "Pasteurization of milk: The heat inactivation kinetics of milk-borne dairy pathogens under commercial-type conditions of turbulent flow". Journal of Dairy Science. 95 (1): 20–35. doi:10.3168/jds.2011-4556. ISSN 0022-0302. PMID 22192181. Archived from the original on 19 July 2022. Retrieved 15 June 2017.
- ↑ "Code of Hygienic Practice for Milk and Milk Products" (PDF). Codex Alimentarius. Archived (PDF) from the original on 23 May 2017. Retrieved 15 June 2017.
- 1 2 Pearce, Lindsay E.; Truong, H. Tuan; Crawford, Robert A.; Yates, Gary F.; Cavaignac, Sonia; Lisle, Geoffrey W. de (1 September 2001). "Effect of Turbulent-Flow Pasteurization on Survival of Mycobacterium avium subsp.paratuberculosis Added to Raw Milk". Applied and Environmental Microbiology. 67 (9): 3964–69. Bibcode:2001ApEnM..67.3964P. doi:10.1128/AEM.67.9.3964-3969.2001. ISSN 0099-2240. PMC 93116. PMID 11525992.
- ↑ "What is double pasteurization?". Archived from the original on 23 April 2021. Retrieved 25 January 2021.
- ↑ Heat Exchangers Archived 18 January 2021 at the Wayback Machine, Tetrapak Diary Processing Handbook
- 1 2 3 Macdonald, Lauren E.; Brett, James; Kelton, David; Majowicz, Shannon E.; Snedeker, Kate; Sargeant, Jan M. (1 November 2011). "A systematic review and meta-analysis of the effects of pasteurization on milk vitamins, and evidence for raw milk consumption and other health-related outcomes". Journal of Food Protection. 74 (11): 1814–32. doi:10.4315/0362-028X.JFP-10-269. ISSN 1944-9097. PMID 22054181.
- 1 2 U.S. Department of Agriculture. 2001. Dietary reference intakes-recommended intakes for individuals. National Academy of Sciences. Institute of Medicine, Food and Nutrition Board. Available at: .
- 1 2 U.S. Department of Agriculture. 2009. "What's in the foods you eat" search tool. Available at: "https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/whats-in-the-foods-you-eat-emsearch-toolem/ Archived 25 April 2017 at the Wayback Machine
- 1 2 Haug, Anna; Høstmark, Arne T; Harstad, Odd M (25 September 2007). "Bovine milk in human nutrition – a review". Lipids in Health and Disease. 6: 25. doi:10.1186/1476-511X-6-25. ISSN 1476-511X. PMC 2039733. PMID 17894873.
- 1 2 Peng, Jing; Tang, Juming; Barrett, Diane M.; Sablani, Shyam S.; Anderson, Nathan; Powers, Joseph R. (22 September 2017). "Thermal pasteurization of ready-to-eat foods and vegetables: Critical factors for process design and effects on quality". Critical Reviews in Food Science and Nutrition. 57 (14): 2970–95. doi:10.1080/10408398.2015.1082126. ISSN 1549-7852. PMID 26529500. S2CID 22614039.
- 1 2 3 Jan, Awsi; Sood, Monika; Sofi, S. A.; Norzom, Tsering (2017). "Non-thermal processing in food applications: A review". International Journal of Food Sciences and Nutrition. 2 (6): 171–180.
- 1 2 3 Sui, Xiaonan; Zhang, Tianyi; Jiang, Lianzhou (25 March 2021). "Soy Protein: Molecular Structure Revisited and Recent Advances in Processing Technologies". Annual Review of Food Science and Technology. Annual Reviews. 12 (1): 119–147. doi:10.1146/annurev-food-062220-104405. ISSN 1941-1413. PMID 33317319. S2CID 229178367.
- ↑ "Gentle pasteurization of milk – with microwaves". ScienceDaily. Archived from the original on 17 May 2018. Retrieved 9 March 2018.
Further reading
- Raw milk expert testimony dated: April 25, 2008 Case: Organic Dairy Company, LLC, and Claravale Farm, Inc., Plaintiffs, vs. No. CU-07-00204 State of California and A.G. Kawamura, Secretary of California Department of Food and Agriculture, – Expert Witnesses: Dr. Theodore Beals & Dr. Ronald Hull
- An alternate view on the alleged safety of pasteurized vs. natural milk from Johns Hopkins University: Realmilk.com, Webmaster (12 August 2015). "The Johns Hopkins Raw Milk Study – A Campaign for Real Milk". A Campaign for Real Milk.
- Unraveling the mysteries of extended shelf life
- Hatch, Sybil E (1 January 2006). Changing our world: true stories of women engineers. Reston, Va.: American Society of Civil Engineers. ISBN 978-0-7844-0841-4. OCLC 62330858.
