| Aryldialkylphosphatase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.1.8.1 | ||||||||
| CAS no. | 117698-12-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Aryldialkylphosphatase (EC 3.1.8.1, also known as phosphotriesterase, organophosphate hydrolase, parathion hydrolase, paraoxonase, and parathion aryl esterase; systematic name aryltriphosphate dialkylphosphohydrolase) is a metalloenzyme that hydrolyzes the triester linkage[1] found in organophosphate insecticides:
| Phosphotriesterase family | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
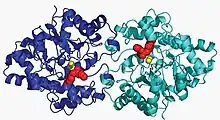 Structure of organophosphorus hydrolase | |||||||||||
| Identifiers | |||||||||||
| Symbol | PTE | ||||||||||
| Pfam | PF02126 | ||||||||||
| InterPro | IPR001559 | ||||||||||
| PROSITE | PDOC01026 | ||||||||||
| SCOP2 | 1dpm / SCOPe / SUPFAM | ||||||||||
| |||||||||||
- an aryl dialkyl phosphate + H2O dialkyl phosphate + an aryl alcohol
The gene (opd, for organophosphate-degrading) that codes for the enzyme is found in a large plasmid (pSC1, 51Kb) endogenous to Pseudomonas diminuta,[2] although the gene has also been found in many other bacterial species such as Flavobacterium sp. (ATCC27551), where it is also encoded in an extrachromosomal element (pSM55, 43Kb).[2]
Organophosphate is the general name for esters of phosphoric acid and is one of the organophosphorus compounds. They can be found as part of insecticides, herbicides, and nerve gases, amongst others. Some less-toxic organophosphates can be used as solvents, plasticizers, and EP additives. The use of organophosphates accounts for approximately 38% of all pesticide use globally.[3]
Gene
Bacterial isolates capable of degrading organophosphate (OP) pesticides have been identified from soil samples from different parts of the world.[3][4] The first organophosphate-degrading bacterial species was isolated from a soil sample from the Philippines in 1973,[5] which identified as Flavobacterium sp. ATCC27551. Since then, other species have demonstrated to have OP-degrading abilities, such as Pseudomonas diminuta (isolated from US soil sample), Agrobacterium radiobacter (isolated from Australian soil sample), Alteromonas haloplanktis (isolated from US soil sample), and Pseudomonas sp. WBC-3 (isolated from Chinese soil sample).[3]
The capacity to hydrolyze organophosphates is not unique to bacteria. A few fungi and cyanobacteria species have been found to also hydrolyze them.[3] Moreover, through sequence homology searches of whole genomes, several other bacterial species were identified that also contain sequences from the same gene family as opd, including pathogenic bacteria such as Escherichia coli (yhfV) and Mycobacterium tuberculosis.[3]
The gene sequence encoding the enzyme (opd) in Flavobacterium sp. ATCC27551 and Pseudomonas diminuta is highly conserved (100% sequence homology),[4] although the plasmids where the genes are found have very different sequences apart from a 5.1Kb[4][6] conserved region where the gene is found.[2]
A closer look on the organization of the opd gene from Flavobacterium suggests a potential transposon-like architecture, which accounts for the widespread distribution of the gene among other microbial species that might have occurred through lateral DNA transfer. The opd gene is flanked by transposition insertion sequences, characteristic of Tn3 family of transposons. Moreover, a transposase-like sequence (homologous to TnpA) and a resolvase-like sequence (homologous to TnpR) were also identified in regions upstream of the opd gene,[4] which are characteristics of class II transposons such as Tn3.
Furthermore, another open reading frame was identified downstream of opd and encodes a protein that further degrades p-nitrophenol, one of the byproducts of OP degradation. This protein is believed to work as a complex with PTE, since a dramatic increase in activity is observed when PTE is present.[4]
Therefore, the characteristic architectural organization of the opd gene region suggests that different species acquired the gene through horizontal transfer through transposition and plasmid transfer.
Protein
Structure
Phosphotriesterase (PTE) belongs to a family metalloenzymes that has two catalytic Zn2+ metal atoms, bridged via a common ligand and coordinated by imidazole side chains of histidine residues that are clustered around the metal atoms.[7] The protein forms a homodimer.[8] The overall structure consists of an α/β-barrel motif, also present in other 20 catalytic proteins. The active sites of these proteins is located at the C-terminal portion of the β-barrel, which is where the active site of PTE is also located.[7]

Catalysis
Catalysis of organophosphates occurs via a nucleophilic substitution with inversion of configuration (SN2 mechanism) about the phosphorus centre of the substrate.[7] In the active site, the metal cations aid in catalysis by further polarizing the P–O bond of the substrate, which makes it more susceptible to a nucleophilic attack. Furthermore, a basic residue abstracts a proton from a water molecule, and the hydroxide ion produced bridges the two divalent cations and acts as the nucleophile. The OH− then attacks the phosphorus centre of the substrate, followed by a proton transfer event. The P–O bond is broken, and the products are released from the active site.[9] The turnover rate (kcat) of phosphotriesterase is nearly 104 s−1 for the hydrolysis of paraoxon,[10] and the products are p-nitrophenol and diethyl phosphoric acid.
Species
Phosphotriesterase is present in two species, Pseudomonas diminuta and Flavobacterium sp. ATCC27551. Other gene variants that also encode organophosphate-degrading enzymes are present in other species. The list includes bacterial species such as the radioresistant Deinococcus radiodurans, pathogens Mycobacterium tuberculosis and Mycobacterium bovis, the anaerobic bacterium Desulfatibacillum alkenivorans, the thermophilic bacteria Geobacillus sp. and Thermoanaerobacter sp. X514, Escherichia coli (yhfV) and many other groups of bacteria,[3] and also some Archaea such as Sulfolobus acidocaldarius.[11]
Subcellular localization
Phosphotriesterase is a membrane-associated protein that is translated with a 29 amino acid-long target peptide (Tat motif),[12][10][13] which is then cleaved from the mature protein after insertion in the plasma membrane.[1] The protein is anchored to the inner membrane of the cell, facing the periplasm.[14]
Function
The enzyme phosphotriesterase hydrolyzes organophosphate compounds by cleaving the triester linkage in the substrate.

The enzyme has a very broad substrate specificity,[12] and is very efficient in catalyzing the reaction: PTE hydrolyzes paraoxon at a rate approaching the diffusion limit,[15] which indicates that the enzyme is optimally evolved for using this substrate.[13] It acts specifically on synthetic organophosphate triesters and phosphorofluoridates.[3] It does not seem to have a natural occurring substrate and may thus have optimally evolved for utilizing paraoxon and other common agricultural pesticides.[15]
The products of the reaction are diethyl phosphoric acid and p-nitrophenol.[4] The latter product is further degraded by an enzyme encoded 750bp downstream of the opd gene, and encodes a 29kDa putative hydrolase that may be involved in degrading aromatic compounds, and works in concert with PTE.[4] This enzyme is homologous to hydrolases in Pseudomonas putida, Pseudomonas azelaica, Rhodococcus sp., and P. fluorescens.[4]
Organophosphates are not toxic to bacteria, but they act as acetylcholinesterase inhibitors in animals.[16] Some species of bacteria are also able to utilize organophosphates as a nutrient and carbon source.[14]
Environmental significance
Phosphotriesterases are considered a strong candidate biocatalyst for bioremediation purposes.[7] Its wide substrate specificity and catalytic efficiency makes it an attractive target for the potential use of microbes containing the opd gene in detoxifying soils that are toxic due to pesticide overuse.[3] Moreover, organophosphates act as acetylcholinesterase (AChE) inhibitors. The AChE neurotransmitter is a vital component of the central nervous system (CNS) in insects in animals, and the inhibition of the proper turnover of this neurochemical results in overstimulation of the CNS, which ultimately results in death of insects and mammals.[3][17] As a result, the use of organophosphate-degrading microorganisms is a potentially effective, low-cost, and environmentally friendly method of removing these toxic compounds from the environment.[3]
History
Bacterial species that had the ability to degrade organophosphate pesticides have been isolated from soil samples from different parts of the world. The first bacterial strain identified to be able to hydrolyze organophosphates was Flavobacterium sp. ATCC 27551, found by Sethunathan and Yoshida in 1973 from a soil sample originally from the Philippines.[5] Since then, other species were found to also have organophosphate-degrading enzymes similar to that found in Flavobacterium[6].
References
- 1 2 Pinjari AB, Pandey JP, Kamireddy S, Siddavattam D (July 2013). "Expression and subcellular localization of organophosphate hydrolase in acephate-degrading Pseudomonas sp. strain Ind01 and its use as a potential biocatalyst for elimination of organophosphate insecticides". Letters in Applied Microbiology. 57 (1): 63–8. doi:10.1111/lam.12080. PMID 23574004. S2CID 12006833.
- 1 2 3 Harper LL, McDaniel CS, Miller CE, Wild JR (October 1988). "Dissimilar plasmids isolated from Pseudomonas diminuta MG and a Flavobacterium sp. (ATCC 27551) contain identical opd genes". Applied and Environmental Microbiology. 54 (10): 2586–9. Bibcode:1988ApEnM..54.2586H. doi:10.1128/AEM.54.10.2586-2589.1988. PMC 204325. PMID 3202637.
- 1 2 3 4 5 6 7 8 9 10 Singh BK (February 2009). "Organophosphorus-degrading bacteria: ecology and industrial applications". Nature Reviews. Microbiology. 7 (2): 156–64. doi:10.1038/nrmicro2050. PMID 19098922. S2CID 205497513.
- 1 2 3 4 5 6 7 8 Siddavattam D, Khajamohiddin S, Manavathi B, Pakala SB, Merrick M (May 2003). "Transposon-like organization of the plasmid-borne organophosphate degradation (opd) gene cluster found in Flavobacterium sp". Applied and Environmental Microbiology. 69 (5): 2533–9. Bibcode:2003ApEnM..69.2533S. doi:10.1128/AEM.69.5.2533-2539.2003. PMC 154515. PMID 12732518.
- 1 2 Sethunathan N, Yoshida T (July 1973). "A Flavobacterium sp. that degrades diazinon and parathion". Canadian Journal of Microbiology. 19 (7): 873–5. doi:10.1139/m73-138. PMID 4727806.
- 1 2 Mulbry WW, Karns JS, Kearney PC, Nelson JO, McDaniel CS, Wild JR (May 1986). "Identification of a plasmid-borne parathion hydrolase gene from Flavobacterium sp. by southern hybridization with opd from Pseudomonas diminuta". Applied and Environmental Microbiology. 51 (5): 926–30. Bibcode:1986ApEnM..51..926M. doi:10.1128/AEM.51.5.926-930.1986. PMC 238989. PMID 3015022.
- 1 2 3 4 Benning MM, Kuo JM, Raushel FM, Holden HM (December 1994). "Three-dimensional structure of phosphotriesterase: an enzyme capable of detoxifying organophosphate nerve agents". Biochemistry. 33 (50): 15001–7. doi:10.1021/bi00254a008. PMID 7999757. S2CID 9424327.
- ↑ Dong YJ, Bartlam M, Sun L, Zhou YF, Zhang ZP, Zhang CG, Rao Z, Zhang XE (October 2005). "Crystal structure of methyl parathion hydrolase from Pseudomonas sp. WBC-3". Journal of Molecular Biology. 353 (3): 655–63. doi:10.1016/j.jmb.2005.08.057. PMID 16181636.
- ↑ Aubert SD, Li Y, Raushel FM (May 2004). "Mechanism for the hydrolysis of organophosphates by the bacterial phosphotriesterase". Biochemistry. 43 (19): 5707–15. doi:10.1021/bi0497805. PMID 15134445.
- 1 2 Mulbry WW, Karns JS (February 1989). "Purification and characterization of three parathion hydrolases from gram-negative bacterial strains". Applied and Environmental Microbiology. 55 (2): 289–93. Bibcode:1989ApEnM..55..289M. doi:10.1128/AEM.55.2.289-293.1989. PMC 184103. PMID 2541658.
- ↑ Chen L, Brügger K, Skovgaard M, Redder P, She Q, Torarinsson E, Greve B, Awayez M, Zibat A, Klenk HP, Garrett RA (July 2005). "The genome of Sulfolobus acidocaldarius, a model organism of the Crenarchaeota". Journal of Bacteriology. 187 (14): 4992–9. doi:10.1128/JB.187.14.4992-4999.2005. PMC 1169522. PMID 15995215.
- 1 2 Classen JJ, Engler CR, Kenerley CM, Whittaker AD (April 2000). "A logistic model of subsurface fungal growth with application to bioremediation". Journal of Environmental Science and Health, Part A. 35 (4): 465–488. doi:10.1080/10934520009376982. S2CID 98035446.
- 1 2 Caldwell SR, Newcomb JR, Schlecht KA, Raushel FM (July 1991). "Limits of diffusion in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta". Biochemistry. 30 (30): 7438–7444. doi:10.1021/bi00244a010. ISSN 0006-2960. PMID 1649628.
- 1 2 Singh BK, Walker A (May 2006). "Microbial degradation of organophosphorus compounds". FEMS Microbiology Reviews. 30 (3): 428–71. doi:10.1111/j.1574-6976.2006.00018.x. PMID 16594965.
- 1 2 Dumas DP, Caldwell SR, Wild JR, Raushel FM (November 1989). "Purification and properties of the phosphotriesterase from Pseudomonas diminuta". The Journal of Biological Chemistry. 264 (33): 19659–65. doi:10.1016/S0021-9258(19)47164-0. PMID 2555328.
- ↑ Lotti M (2002). "Promotion of organophosphate induced delayed polyneuropathy by certain esterase inhibitors". Toxicology. 181–182: 245–248. doi:10.1016/s0300-483x(02)00291-3. PMID 12505319.
- ↑ Ragnarsdottir KV (2000). "Environmental fate and toxicology of organophosphate pesticides". Journal of the Geological Society. 157 (4): 859–876. Bibcode:2000JGSoc.157..859R. doi:10.1144/jgs.157.4.859. S2CID 129950334.
Further reading
- Aldridge WN (January 1953). "Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination". The Biochemical Journal. 53 (1): 110–7. doi:10.1042/bj0530110. PMC 1198110. PMID 13032041.
- Bosmann HB (July 1972). "Membrane marker enzymes. Characterization of an arylesterase of guinea pig cerebral cortex utilizing p-nitrophenyl acetate as substrate". Biochimica et Biophysica Acta. 276 (1): 180–91. doi:10.1016/0005-2744(72)90019-8. PMID 5047702.
- Mackness MI, Thompson HM, Hardy AR, Walker CH (July 1987). "Distinction between 'A'-esterases and arylesterases. Implications for esterase classification". The Biochemical Journal. 245 (1): 293–6. doi:10.1042/bj2450293. PMC 1148115. PMID 2822017.
- Main AR (1960). "The differentiation of the A-type esterases in sheep serum". Biochem. J. 75 (1): 188–195. doi:10.1042/bj0750188. PMC 1204348. PMID 14420012.