 | |
| Names | |
|---|---|
| IUPAC name
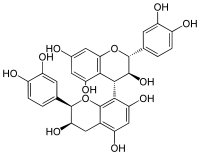
[(2R,3S,4S)-Flavan-3,3′,4′,5,7-pentol]-(4→8)-[(2R,3R)-flavan-3,3′,4′,5,7-pentol] | |
| Systematic IUPAC name
(2R,2′R,3S,3′R,4S)-2,2′-Bis(3,4-dihydroxyphenyl)-3,3′,4,4′-tetrahydro-2H,2′H-[4,8′-bi-1-benzopyran]-3,3′,5,5′,7,7′-hexol | |
| Other names
Procyanidin B4 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H26O12 | |
| Molar mass | 578.52 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Procyanidin B4 is a B type proanthocyanidin.
Procyanidin-B4 is a catechin-(4α→8)-epicatechin dimer. It is found in the litchi pericarp,[1] in grape seeds,[2] and, along with 4-cis-isomer of procyanidin B4, in beer.[3]
See also
References
- ↑ Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn.) pericarp. Mouming Zhao; Bao Yang; Jinshui Wang; Yang Liu; Limei Yu; Yueming Jiang, 2007
- ↑ Catechin and proanthocyanidin B4 from grape seeds prevent doxorubicin-induced toxicity in cardiomyocytes. Yu Du and Hongxiang Lou, 2008.
- ↑ Structure elucidation of proanthocyanidins: Direct synthesis and isolation from Pilsener beer. Jan Delcour, 1985 Archived 2011-07-06 at the Wayback Machine
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.