| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
3,4-Dihydroxycyclobut-3-ene-1,2-dione | |||
| Other names
Quadratic acid Cyclobutenedioic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.018.875 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H2O4 | |||
| Molar mass | 114.056 g·mol−1 | ||
| Appearance | white crystalline powder | ||
| Melting point | > 300 °C (572 °F; 573 K) | ||
| Acidity (pKa) | pKa1 = 1.5 pKa2 = 3.4 | ||
| Hazards[2] | |||
| GHS labelling: | |||
 | |||
| Danger | |||
| H314 | |||
| P260, P280, P301+P330+P331, P303+P361+P353, P304+P340+P310, P305+P351+P338 | |||
| Flash point | 190 °C (374 °F; 463 K)[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
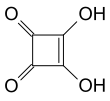
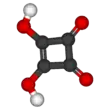
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.[4]
The conjugate base of squaric acid is the hydrogensquarate anion HC4O−4; and the conjugate base of the hydrogensquarate anion is the divalent squarate anion C4O2−4. This is one of the oxocarbon anions, which consist only of carbon and oxygen.
Squaric acid is a reagent for chemical synthesis, used for instance to make photosensitive squaraine dyes and inhibitors of protein tyrosine phosphatases.
Chemical properties
Squaric acid is a white crystalline powder.[5] The onset of thermal decomposition depends on the different thermodynamic conditions such as heating rates.
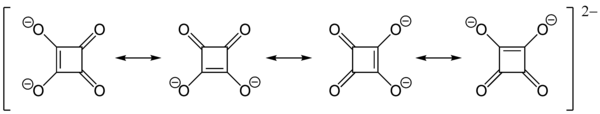
The structure of squaric acid is not a perfect square, as the carbon–carbon bond lengths are not quite equal. The high acidity with pKa1 = 1.5 for the first proton and pKa2 = 3.4 for the second is attributable to resonance stabilization of the anion.[6] Because the negative charges are equally distributed between each oxygen atom, the dianion of squaric acid is completely symmetrical (unlike squaric acid itself) with all C−C bond lengths identical and all C−O bond lengths identical.

Derivatives
Many of the reactions of squaric acid involve the OH groups. The molecule behaves similarly to a strong dicarboxylic acid. It is stronger acid than typical carboxylic acids.[7]
- C4O2(OH)2 → [C4O3(OH)]− + H+, pKa1 = 1.5
- [C4O3(OH)]− → [C4O4]2− + H+, pKa2 = 3.5
The OH groups are labile in squaric acid. It forms a dichloride with thionyl chloride:
- C4O2(OH)2 + 2 SOCl2 → C4O2Cl2 + 2 HCl + 2 SO2
The chlorides are good leaving groups, reminiscent of acid chlorides. They are displaced by diverse nucleophiles. In this way dithiosquarate can be prepared.[8]
The bis(methylether) is prepared by alkylation with trimethyl orthoformate.[9]
Dibutyl squarate is used for the treatment of warts[10] and for alopecia areata .[11]
Diethyl squarate has been used as an intermediate in the synthesis of perzinfotel.
Squaramides are prepared by displacement of alkoxy or chloride groups from C4O2X2 (X = OR, Cl).[8][12]
One or both of the oxygen (=O) groups in the squarate anion can be replaced by dicyanomethylene =C(CN)2. The resulting anions, such as 1,2-bis(dicyanomethylene)squarate and 1,3-bis(dicyanomethylene)squarate, retain the aromatic character of squarate and have been called pseudo-oxocarbon anions.
Photolysis of squaric acid in a solid argon matrix at 10 K (−263 °C) affords acetylenediol.[13]
Coordination complexes
Squarate dianion behaves similarly to oxalate, forming mono- and polynuclear complexes with hard metal ions. Cobalt(II) squarate hydrate Co(C4O4)·2H2O (yellow, cubic) can be prepared by autoclaving cobalt(II) hydroxide and squaric acid in water at 200 °C. The water is bound to the cobalt atom, and the crystal structure consists of a cubic arrangement of hollow cells, whose walls are either six squarate anions (leaving a 7 Å wide void) or several water molecules (leaving a 5 Å void).[14]
Cobalt(II) squarate dihydroxide Co3(OH)2(C4O4)2·3H2O (brown) is obtained together with the previous compound. It has a columnar structure including channels filled with water molecules; these can be removed and replaced without destroying the crystal structure. The chains are ferromagnetic; they are coupled antiferromagnetically in the hydrated form, ferromagnetically in the anhydrous form.[14]
Copper(II) squarate monomeric and dimeric mixed-ligand complexes were synthesized and characterized.[15] Infrared, electronic and Q-Band EPR spectra as well as magnetic susceptibilities are reported.
The same method yields iron(II) squarate dihydroxide Fe2(OH)2(C4O4) (light brown).[14]
Synthesis
The original synthesis started with the ethanolysis of perfluorocyclobutene to give 1,2-diethoxy-3,3,4,4-tetrafluoro-1-cyclobutene. Hydrolysis gives the squaric acid.[16][4]
Although impractical, squarate and related anions such as deltate C3O2−3 and acetylenediolate C2O2−2 are obtainable by reductive coupling of carbon monoxide using organouranium complexes.[17][18]
See also
- Acetylenediol, H2(CO)2 or HO−C≡C−OH
- Deltic acid, H2(CO)3
- Croconic acid, H2(CO)5
- Rhodizonic acid, H2(CO)6
- Cyclopropenone, C3H2O
- Cyclobutene, C4H6
- Squaramide, C4O2(NH2)2, a nitrogen analog of squaric acid, where the OH groups of squaric acid are replaced by NH2 groups
- Moniliformin, NaC4HO3, the sodium salt of semisquaric acid
References
- ↑ 3,4-Dihydroxy-3-cyclobutene-1,2-dione. Sigma-Aldrich
- ↑ "SICHERHEITSDATENBLATT". 21 March 2021.
- ↑ 3,4-Dihydroxy-3-cyclobutene-1,2-dione, 98+%. Alfa Aesar
- 1 2 Robert West (1980). "History of the Oxocarbons". In Robert West (ed.). Oxocarbons. Academic Press. pp. 1–14. doi:10.1016/B978-0-12-744580-9.50005-1. ISBN 9780127445809.
- ↑ Lee, K.-S.; Kweon, J. J.; Oh, I.-H.; Lee, C. E. (2012). "Polymorphic phase transition and thermal stability in squaric acid (H
2C
4O
4)". J. Phys. Chem. Solids. 73 (7): 890–895. doi:10.1016/j.jpcs.2012.02.013. - ↑ West, Robert; Powell, David L. (1963). "New Aromatic Anions. III. Molecular Orbital Calculations on Oxygenated Anions". J. Am. Chem. Soc. 85 (17): 2577–2579. doi:10.1021/ja00900a010.
- ↑ "Acidity Tables for Heteroatom Organic Acids and Carbon Acids".
- 1 2 Arthur H. Schmidt (1980). "Reaktionen von Quadratsäure und Quadratsäure-Derivaten". Synthesis. 1980 (12): 961. doi:10.1055/s-1980-29291.
- ↑ Liu, Hui; Tomooka, Craig S.; Xu, Simon L.; Yerxa, Benjamin R.; Sullivan, Robert W.; Xiong, Yifeng; Moore, Harold W. (1999). "Dimethyl Squarate and ITS Conversion to 3-Ethenyl-4-Methoxycyclobutene-1,2-Dione and 2-Butyl-6-Ethenyl-5-Methoxy-1,4-Benzoquinone". Organic Syntheses. 76: 189. doi:10.15227/orgsyn.076.0189.
- ↑ Silverberg, Nanette B.; Lim, Joseph K.; Paller, Amy S.; Mancini, Anthony J. (2000). "Squaric acid immunotherapy for warts in children". Journal of the American Academy of Dermatology. 42 (5): 803–808. doi:10.1067/mjd.2000.103631. PMID 10775858.
- ↑ Yoshimasu, Takashi; Furukawa, Fukumi (2016). "Modified immunotherapy for alopecia areata". Autoimmunity Reviews. 15 (7): 664–667. doi:10.1016/j.autrev.2016.02.021. PMID 26932732.
- ↑ Ian Storer, R.; Aciro, Caroline; Jones, Lyn H. (2011). "Squaramides: Physical Properties, Synthesis and Applications". Chem. Soc. Rev. 40 (5): 2330–2346. doi:10.1039/c0cs00200c. PMID 21399835.
- ↑ Maier, Günther; Rohr, Christine (1995). "Ethynediol: Photochemical generation and matrix-spectroscopic identification". Liebigs Annalen. 1996 (3): 307–309. doi:10.1002/jlac.199619960303.
- 1 2 3 Hitoshi, Kumagai; Hideo, Sobukawa; Mohamedally, Kurmoo (2008). "Hydrothermal syntheses, structures and magnetic properties of coordination frameworks of divalent transition metals". Journal of Materials Science. 43 (7): 2123–2130. Bibcode:2008JMatS..43.2123K. doi:10.1007/s10853-007-2033-8. S2CID 95205908.
- ↑ Reinprecht, J. T.; Miller, J. G.; Vogel, G. C.; et al. (1979). "Synthesis and Characterization of Copper(II) Squarate Complexes". Inorg. Chem., 19, 927-931
- ↑ Park, J. D.; Cohen, S. & Lacher, J. R. (1962). "Hydrolysis Reactions of Halogenated Cyclobutene Ethers: Synthesis of Diketocyclobutenediol". J. Am. Chem. Soc. 84 (15): 2919–2922. doi:10.1021/ja00874a015.
- ↑ Frey, Alistair S.; Cloke, F. Geoffrey N.; Hitchcock, Peter B. (2008). "Mechanistic Studies on the Reductive Cyclooligomerisation of CO by U(III) Mixed Sandwich Complexes; the Molecular Structure of [(U(η-C8H6{Si′Pr3-1,4}2)(η-Cp*)]2(μ-η1:η1-C2O2)". Journal of the American Chemical Society. 130 (42): 13816–13817. doi:10.1021/ja8059792. PMID 18817397.
- ↑ Summerscales, Owen T.; Frey, Alistair S. P.; Cloke, F. Geoffrey N.; Hitchcock, Peter B. (2009). "Reductive disproportionation of carbon dioxide to carbonate and squarate products using a mixed-sandwich U(III) complex". Chemical Communications (2): 198–200. doi:10.1039/b815576c. PMID 19099067.