| Kidney cancer | |
|---|---|
| Other names | Renal cancer |
 | |
| Micrograph showing the most common type of kidney cancer (clear cell renal cell carcinoma). H&E stain. | |
| Specialty | Oncology nephrology Urology |
| Symptoms | Blood in the urine, lump in the abdomen, back pain[1][2][3] |
| Usual onset | After the age of 45[4] |
| Types | Renal cell carcinoma (RCC), transitional cell carcinoma (TCC), Wilms tumor[4] |
| Risk factors | Smoking, certain pain medications, previous bladder cancer, being overweight, high blood pressure, certain chemicals, family history[1][2] |
| Diagnostic method | Tissue biopsy[1][2][3] |
| Treatment | Surgery, radiation therapy, chemotherapy, immunotherapy, targeted therapy[1][2][3] |
| Prognosis | Five-year survival ~75% (US 2015)[4] |
| Frequency | 403,300 (2018)[5] |
| Deaths | 175,000[5] |
Kidney cancer, also known as renal cancer, is a group of cancers that starts in the kidney.[4] Symptoms may include blood in the urine, a lump in the abdomen, or back pain.[1][2][3] Fever, weight loss, and tiredness may also occur.[1][2][3] Complications can include spread to the lungs or brain.[6]
The main types of kidney cancer are renal cell cancer (RCC), transitional cell cancer (TCC), and Wilms' tumor.[7] RCC makes up approximately 80% of kidney cancers, and TCC accounts for most of the rest.[8] Risk factors for RCC and TCC include smoking, certain pain medications, previous bladder cancer, being overweight, high blood pressure, certain chemicals, and a family history.[1][2] Risk factors for Wilms' tumor include a family history and certain genetic disorders such as WAGR syndrome.[3] Diagnosis may be suspected based on symptoms, urine testing, and medical imaging.[1][2][3] It is confirmed by tissue biopsy.[1][2][3]
Treatment may include surgery, radiation therapy, chemotherapy, immunotherapy, and targeted therapy.[1][2][3] Kidney cancer newly affected about 403,300 people and resulted in 175,000 deaths globally in 2018.[5] Onset is usually after the age of 45.[4] Males are affected more often than females.[4] The overall five-year survival rate is 75% in the United States, 71% in Canada, 70% in China, and 60% in Europe.[4][9][10][11] For cancers that are confined to the kidney, the five-year survival rate is 93%, if it has spread to the surrounding lymph nodes it is 70%, and if it has spread widely, it is 12%.[4] Kidney cancer has been identified as the 13th most common form of cancer,[12] and is responsible for 2% of the world's cancer cases and deaths.[13] The incidence of kidney cancer has continued to increase since 1930. Renal cancer is more commonly found in populations of urban areas than rural areas.[14]
Signs and symptoms
Early on, kidney masses do not typically cause any symptoms and are undetectable on physical examination.[15] As kidney cancer becomes more advanced it classically results in blood in the urine, flank or back pain, and a mass.[15] Other symptoms that are consistent with advanced disease include weight loss, fever, night sweats, palpable swollen lymph nodes in the neck, non-reducing varicocele, bone pain, continuous cough, and bilateral lower leg swelling.[15][16][17]
The classic triad of visible blood in the urine (hematuria), flank pain and palpable abdominal mass occurs in less than 15% of the cases. RCC may present with signs and symptoms caused by the substances the cancer cell produce (i.e. paraneoplastic syndromes).
Paraneoplastic syndromes caused by kidney cancer can be broadly classified as endocrine and non-endocrine. Endocrine dysfunctions include increase in blood calcium levels (hypercalcemia), high blood pressure (hypertension), increased red bloods (polycythemia), liver dysfunction, milky nipple discharge unrelated normal breast-feeding (galactorrhea), and Cushing's syndrome. Non-endocrine dysfunctions include deposition of protein in tissue (amyloidosis), decrease in hemoglobin or red blood cells (anemia), disorders of nerves, muscles (neuromyopathies), blood vessels (vasculopathy) and blood clotting mechanisms (coagulopathy).[18]
Causes
Factors that increase the risk of kidney cancer include smoking, high blood pressure, obesity, faulty genes, a family history of kidney cancer, having kidney disease that needs dialysis, being infected with hepatitis C, and previous treatment for testicular cancer or cervical cancer.[19][20]
There are also other possible risk factors such as kidney stones being investigated.[21][22]
About 25-30% of kidney cancer is attributed to smoking.[20] Smokers have a 1.3 times higher risk of developing kidney cancer compared to non-smokers. Moreover, there is a dose-dependent increased risk of cancer development. Men who smoke more than 20 cigarettes per day have twice the risk. Likewise, women who smoke more than 20 cigarettes per day have 1.5 times the risk of non-smokers. After 10 years of smoking cessation a substantial reduction is seen in the risk of developing kidney cancer.[23]
Diagnosis
Due to the increase in ultrasound and CT imaging for nonspecific abdominal complaints, kidney masses are frequently incidentally diagnosed on medical imaging.[15][24][25] More than 60% of renal cell carcinoma (the most common type of kidney cancer), are diagnosed incidentally by abdominal imaging for nonspecific abdominal complaints.[15][26]
Kidney masses can be classified by the nature of the cells in the growth, or by its appearance on radiography.[15] The term cancer refers to a malignant tumor, which is an uncontrolled growth of abnormal cells.[27] However, kidney masses can be due to growth of normal tissue (benign), inflammatory (a reaction of the immune system), or vascular (cells of the blood vessels).
Medical imaging
Since there is a large differential diagnosis for a kidney tumor, the first step is to characterize the mass with medical imaging to assess its likelihood of being benign or malignant. Ultrasonography is sometimes used to evaluate a suspected kidney mass, as it can characterize cystic and solid kidney masses without radiation exposure and at relative low cost.[15] Radiologically tumors are grouped based on appearance into simple cystic, complex cystic, or solid.[15] The most important differentiating feature of a cancerous and non-cancerous tumor on imaging is enhancement.[28] Simple cysts, which are defined by strict criteria[29] are safe to be monitored if the person does not have any symptoms.[15] However, all masses that are not clearly simple cysts should be further evaluated and confirmed by alternate imaging techniques.[30][15]
Computed tomography (CT) of the abdomen administered with and without IV contrast is the ideal imaging to diagnose and stage kidney cancer.[31][30][15] There is tentative evidence that iodinated contrast agents may cause worsening of kidney function in people with chronic kidney disease (CKD) with a glomerular filtration rate (GFR) less than 45ml/min/1.73m2 and should therefore be given cautiously in this group.[32]
Abdominal magnetic resonance imaging (MRI) is an alternative imaging method that can be used to characterize and stage a kidney mass.[31][30][15] It may be suggested if contrast material cannot be given.[31] MRI can also evaluate the inferior vena cava if the mass is suspected to extend outside the kidney.[31]
Since the lungs are the most common organ for kidney cancer to spread to, a chest X-ray or CT scan may be ordered based on the person's risk for metastatic disease.[15][30]
Histopathologic classification

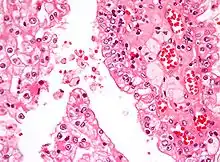
The most common type of kidney malignancy is renal cell carcinoma,[33] which is thought to originate from cells in the proximal convoluted tubule of the nephron.[15][34] Another type of kidney cancer although less common, is transitional cell cancer (TCC) or urothelial carcinoma of the renal pelvis.[35] The renal pelvis is the part of the kidney that collects urine and drains it into a tube called the ureter.[35] The cells that line the renal pelvis are called transitional cells, and are also sometimes called urothelial cells. The transitional/urothelial cells in the renal pelvis are the same type of cells that line the ureter and bladder. For this reason TCC of the renal pelvis is distinct from RCC and is thought to behave more like bladder cancer.[35] Other rare types of kidney cancers that can arise from the urothelial cells of the renal pelvis are squamous cell carcinoma and adenocarcinoma.[15]
Other causes of kidney cancer include the following:[15]
- Sarcoma- for example leiomyosarcoma, liposarcoma, angiosarcoma, clear-cell sarcoma and rhabdomyosarcoma are types of sarcomas that have occurred in the kidney
- Metastatic tumor from distant organ
- Lymphoma
- Wilms tumor- an embryonic tumor that is the most common type of kidney cancer in children
- Carcinoid tumor of the renal pelvis[36]
- Carcinosarcoma[37]
- Inverted urothelial papilloma- was traditionally regarded as a benign growth. However, there may be an increased risk for recurrence and transformation to TCC.[38]
In children, Wilms tumor is the most common type of kidney cancer.[15] Mesoblastic nephroma, although rare, also typically presents in childhood.
Renal cell carcinoma has been further divided into sub-types based on histological features and genetic abnormalities. The 2004 WHO Classification of the Renal Tumors of the Adults describes these categories:[39]
- Clear cell RCC
- Multilocular clear cell RCC
- Papillary RCC
- Chromophobe RCC
- Carcinoma of the collecting ducts of Bellini
- Renal medullary carcinoma
- Xp11 translocation carcinomas
- Carcinoma associated with neuroblastoma
- Mucinous tubular and spindle cell carcinoma
- Mixed epithelial stromal tumor[40]
Tumors that are considered benign include angiomyolipoma, oncocytoma, reninoma (juxtaglomerular cell tumor), and renal adenoma.[15]
Immunohistochemistry
| PAX8 | CD10 | CAIX | RCC | Melanocytic markers | Vimentin | CK7 | HMWCK | CD117 / KIT | AMACR | GATA3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clear cell RCC | + | + | + (box-like) | + | - | + | - | - | - | -/+ | - |
| Papillary RCC | + | + | +/- | + | - | + | + | - | - | + | - |
| Clear cell papillary RCC | + | - | + (cup-like) | +/- | - | + | + | +/- | - | - | +/- |
| Chromophobe RCC | + | -/+ | - | +/- | - | - | + | - | + | - | +/- |
| Oncocytoma | + | -/+ | - | - | - | - | Focal | - | + | -/+ | - |
| Angiomyolipoma | - | - | -/+ | - | + | -/+ | - | - | - | - | - |
| Collecting duct carcinoma | + | - | -/+ | - | - | + | +/- | + | - | - | - |
| Tubulocystic carcinoma | + | + | +/- | + | - | +/- | -/+ | - | - | +/- | - |
| Translocation RCC | + | +/- | -/+ | +/- | +/- | + | - | - | - | +/- | - |
| MTSCC | + | -/+ | -/+ | +/- | - | + | + | -/+ | - | +/- | - |
Legend:
| |||||||||||
Laboratory studies
People with suspected kidney cancer should also have their kidney function evaluated to help determine treatment options. Blood tests to determine kidney function include a comprehensive metabolic panel (CMP), a complete blood count (CBC).[43][30] In addition, these tests help understand the overall health of the person, which can be affected by metastatic disease (disease that is outside of the kidney). For example, liver or bone involvement could result in abnormal liver enzymes, electrolyte abnormalities, or anemia. A urine sample should also be collected for urinalysis.[30][15]
Biopsy
The utility of renal mass biopsy (RMB) lies in that it can confirm malignancy with reliability, can direct therapy based on diagnosis, and can provide drainage.[30]
Once imaging has been completed, renal mass biopsy should be considered if there is a high likelihood that the mass is hematologic, metastatic, inflammatory, or infectious.[30] These types of lesions would not be managed surgically, differing from cancer originating from the kidney. Cancer originating outside the kidney and lymphoma are managed systemically.[15][30]
RMB can accurately diagnose malignancy, however, it cannot reliably diagnose benign disease. In other words, if the biopsy shows cancer, there is a 99.8% chance that kidney cancer is present (Positive Predictive Value= 99.8%). A negative biopsy does not rule out a diagnosis of cancer.[44]
Staging
Staging is the process that helps determine the extent and spread of the disease.[45] Renal cell carcinoma is the only type of kidney cancer that can be staged. The first step of staging follows the TNM staging system proposed by the Union International Contre le Cancer that is widely used among cancers in other organs.[15] The TNM staging system classifies the primary tumor (T), lymph nodes (N) and distant metastasis (M) of the disease. The American Joint Committee on Cancer (AJCC) published a Cancer Staging Manual revision in 2010 that describes the values of TMN for renal cell carcinoma.[46][15]
Lymph node involvement is classified as either regional lymph node metastasis (N1), or no involvement (N0).[46] Similarly, M1 describes distant metastasis, while M0 describes no distant metastasis.[46]
The primary tumor of renal cell carcinoma is categorized in the table below, as according to the AJCC 8th Edition Cancer Staging Manual:[47][48]
| Stage | TNM | Description |
|---|---|---|
| Tx, N0, M0 | Tumor cannot be assessed | |
| T0, N0, M0 | No evidence of primary tumor | |
| I | T1, N0, M0 | Tumor ≤7 cm; limited to kidney |
| T1a, N0, M0 | Tumor ≤4 cm; limited to kidney | |
| T1b, N0, M0 | Tumor 4-≤7 cm; limited to kidney | |
| II | T2, N0, M0 | Tumor >7 cm; limited to kidney |
| T2a, N0, M0 | Tumor 7-≤10 cm; limited to kidney | |
| T2b, N0, M0 | Tumor >10 cm; limited to kidney | |
| III | T3, N0, M0 | Tumor extends to major veins or perinephric tissue but not into ipsilateral adrenal gland nor beyond Gerota's fascia |
| T3a, N0, M0 | Tumor grossly extends into renal vein or its segmental branches, or tumor invades the pelvicalyceal system, or tumor invades perirenal and/or renal sinus fat but not beyond Gerota's fascia | |
| T3b, N0, M0 | Tumor grossly extends into vena cava below the diaphragm | |
| T3c, N0, M0 | Tumor grossly extends into vena cava above the diaphragm or invades the wall of the vena cava | |
| T1-T3, N1, M0 | The main tumor can be any size and may be outside the kidney, but it has not spread beyond Gerota’s fascia. The cancer has spread to regional lymph nodes (N1) but has not spread to distant lymph nodes or other organs (M0). | |
| IV | T4, any N, M0 | Tumor invades beyond Gerota's fascia |
| Any T, any N, M1 | Tumor has spread to distant lymph nodes and/or other organs. |
The lungs are the most common site for metastasis,[30] with other common sites including bone, brain, liver, adrenal gland and distant lymph nodes.[43][49][46]
 Stage 1 kidney cancer
Stage 1 kidney cancer Stage 2 kidney cancer
Stage 2 kidney cancer Stage 3 kidney cancer
Stage 3 kidney cancer Stage 4 kidney cancer
Stage 4 kidney cancer
Treatment
Treatment for kidney cancer depends on the type and stage of the disease. Surgery is the most common treatment as kidney cancer does not often respond to chemotherapy and radiotherapy. Surgical complexity can be estimated by the RENAL Nephrometry Scoring System. If the cancer has not spread it will usually be removed by surgery. In some cases this involves removing the whole kidney however most tumors are amenable to partial removal to eradicate the tumor and preserve the remaining normal portion of the kidney. Surgery is not always possible – for example, the patient may have other medical conditions that prevent it, or the cancer may have spread around the body and doctors may not be able to remove it.[50] If the cancer cannot be treated with surgery other techniques such as freezing the tumour or treating it with high temperatures may be used. However, these are not yet used as standard treatments for kidney cancer.[51] Recently, evidence stemming from the KEYNOTE-564 study has shed light on the potential use of systemic therapy in the adjuvant setting, with promising results. Patients exhibiting specific clear cell RCC tumor characteristics and having undergone treatment with Pembrolizumab for 17 cycles (around 1 year) had significant improvement in disease-free survival. However, the study has yet to yield conclusive findings in relation to overall survival. [52]
Other treatment options include biological therapies such as everolimus, torisel, nexavar, sutent, and axitinib, the use of immunotherapy including interferon and interleukin-2.[53][54][55] Immunotherapy is successful in 10 to 15% of people.[56] Sunitinib is the current standard of care in the adjuvant setting along with pazopanib; these treatments are often followed by everolimus, axitinib, and sorafenib. Immune checkpoint inhibitors are also in trials for kidney cancer, and some have gained approval for medical use.[57]
In the second line setting, nivolumab demonstrated an overall survival advantage in advanced clear renal cell carcinoma over everolimus in 2015 and was approved by the FDA.[57][58] Cabozantinib also demonstrated an overall survival benefit over everolimus and was approved by the FDA as a second-line treatment in 2016.[59][60][61] Lenvatinib in combination with everolimus was approved in 2016 for patients who have had exactly one prior line of angiogenic therapy.[62]
In Wilms' tumor, chemotherapy, radiotherapy and surgery are the accepted treatments, depending on the stage of the disease when it is diagnosed.[63]
Children
The majority of kidney cancers reported in children are Wilms' tumors. These tumors can begin to grow when a fetus is still developing in the uterus, and may not cause problems until the child is a few years old. Wilms' tumor is most common in children under the age of 5, but can rarely be diagnosed in older children or in adults. It is still not clear what causes most Wilms' tumors. The most common symptoms are swelling of the abdomen and blood in the urine.[63]
Epidemiology
Around 208,500 new cases of kidney cancer are diagnosed in the world each year, accounting for just under 2% of all cancers.[64] The highest rates are recorded in North America and the lowest rates in Asia and Africa.[65]
Lifestyle risk factors
Certain lifestyle factors have been associated with the development of renal cancer, although not all of them can be considered definitive causes. These include smoking, chemical carcinogens, radiation, viruses, diet and obesity, hypertension, diuretics,[14] and alcohol consumption.[12] Only a small percentage of kidney cancer cases have been linked to genetic factors.[12] With obesity listed as one of the risk factors, daily physical activity and engaging in a healthy diet is proven to lower the rates of developing kidney cancer in the future.[14]
Age
The incidence rate of renal cancer increases with the age of an individual, with 75 being the approximate age of the peak incidence rate, as of 2018.[12] However, nearly one half of all cases are diagnosed before the age of 65.[12] In both male and female children, renal tumors represent 2% to 6% of kidney cancer, with Wilms' tumor[14] being the most common.
Sex
The incidence of kidney cancer is two times greater in men than in women, and this is thought to be due to biological differences. Mortality rates typically decrease more rapidly in women compared to men.[12]
International variations
Incidence rates of kidney cancer can vary throughout the world. As of 2018, Czech Republic and Lithuania have the highest incidence rate of kidney cancer worldwide, with an age-standardized rate of 21.9/100,000 in males (Czech Republic) and 18.7/100,000 in males (Lithuania.) China, Thailand, and African countries (low-risk countries) have an incidence rate that is less than 2/100,000.[12]
Since the early 2000s, Austria and Poland have been the only countries to report a decrease in kidney cancer rates.[12]
Diagnosis access bias plays a large role in the epidemiology of kidney cancer. Differences in kidney cancer diagnosis across regions are likely due to differences in healthcare access, rather than a population's biological factors. Discrepancies in kidney cancer diagnosis has most likely led to the underrepresentation of mortality and incidence in low income countries.
Race
Race and ethnicity may be a factor in the distribution of kidney cancer around the United States. There are higher incidence rates in Black men and Hispanics, an average rate for American Indians, and low rates in Asians in the United States. Black people with kidney cancer have lower mortality rates than Caucasians in the United States.[12]
Screening
Accessibility for cancer screening is not very common due to high expenses. Improving cancer registries can improve care to those who have kidney cancer as well as decreasing the incidence and death rates. Safe and dependable treatment is key with the screening and treatment, which is not always the case in many developing nations.[66]
United States
The United States' NIH estimates for 2013 around 64,770 new cases of kidney cancer and 13,570 deaths from the disease.[67]
The incidence of kidney cancer is also increasing in the United States. This is thought to be a real increase, not only due to changes in the way the disease is diagnosed.[68]
Europe
The most recent estimates of incidence of kidney cancer suggest that there are 63,300 new cases annually in the EU25. In Europe, kidney cancer accounts for nearly 3% of all cancer cases.[69] Kidney cancer is the eighth most common cancer in the UK (around 10,100 people were diagnosed with the disease in 2011), and it is the fourteenth most common cause of cancer death (around 4,300 people died in 2012).[70]
References
- 1 2 3 4 5 6 7 8 9 10 "Renal Cell Cancer Treatment". National Cancer Institute. 2019. Retrieved 8 June 2019.
- 1 2 3 4 5 6 7 8 9 10 "Transitional Cell Cancer (Kidney/Ureter) Treatment". National Cancer Institute. 2019. Retrieved 8 June 2019.
- 1 2 3 4 5 6 7 8 9 "Wilms Tumor and Other Childhood Kidney Tumors Treatment". National Cancer Institute. 2019. Retrieved 8 June 2019.
- 1 2 3 4 5 6 7 8 "Cancer of the Kidney and Renal Pelvis - Cancer Stat Facts". SEER. Retrieved 30 May 2019.
- 1 2 3 "Cancer today". IARC. Retrieved 30 May 2019.
- ↑ Sommers MS, Fannin E (2014). Diseases and Disorders: A Nursing Therapeutics Manual. F.A. Davis. p. 657. ISBN 9780803644878.
- ↑ "Kidney Cancer". National Cancer Institute. 2019. Retrieved 8 June 2019.
- ↑ Mulders PF, Brouwers AH, Hulsbergen-van der Kaa CA, van Lin EN, Osanto S, de Mulder PH (February 2008). "[Guideline 'Renal cell carcinoma']". Ned Tijdschr Geneeskd (in Dutch). 152 (7): 376–80. PMID 18380384.
- ↑ "Survival statistics for kidney cancer - Canadian Cancer Society". www.cancer.ca. Retrieved 2019-12-02.
- ↑ "European Network of Cancer Registeries" (PDF).
- ↑ Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N (2018). "Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries". The Lancet Global Health. 6 (5): e555–e567. doi:10.1016/S2214-109X(18)30127-X. hdl:10072/386179. PMID 29653628.
- 1 2 3 4 5 6 7 8 9 Scelo G, Larose TL (2018-12-20). "Epidemiology and Risk Factors for Kidney Cancer". Journal of Clinical Oncology. 36 (36): 3574–3581. doi:10.1200/JCO.2018.79.1905. ISSN 0732-183X. PMC 6299342. PMID 30372394.
- ↑ Padala SA, Barsouk A, Thandra KC, Saginala K, Mohammed A, Vakiti A, Rawla P, Barsouk A (June 2020). "Epidemiology of Renal Cell Carcinoma". World Journal of Oncology. 11 (3): 79–87. doi:10.14740/wjon1279. ISSN 1920-4531. PMC 7239575. PMID 32494314.
- 1 2 3 4 Pascual D, Borque A (2008-11-04). "Epidemiology of Kidney Cancer". Advances in Urology. 2008: e782381. doi:10.1155/2008/782381. ISSN 1687-6369. PMC 2581742. PMID 19009036.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Campbell SC, Lane BR (2012). "Malignant Renal Tumors". In Wein AJ, Kavoussi LR (eds.). Campbell-Walsh Urology. Elselvier. ISBN 978-1455775675.
- ↑ Hidayat K, Du X, Zou SY, Shi BM (July 2017). "Blood pressure and kidney cancer risk: meta-analysis of prospective studies". Journal of Hypertension. 35 (7): 1333–1344. doi:10.1097/HJH.0000000000001286. PMID 28157813. S2CID 3454741.
- ↑ Professionals S. "EAU Guidelines: Renal Cell Carcinoma". Uroweb. Retrieved 2019-12-03.
- ↑ Palapattu GS, Kristo B, Rajfer J (2002). "Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma". Reviews in Urology. 4 (4): 163–170. ISSN 1523-6161. PMC 1475999. PMID 16985675.
- ↑ Tahbaz R, Schmid M, Merseburger AS (2018). "Prevention of kidney cancer incidence and recurrence: lifestyle, medication and nutrition". Current Opinion in Urology. 28 (1): 62–79. doi:10.1097/MOU.0000000000000454. ISSN 1473-6586. PMID 29059103. S2CID 25998957.
- 1 2 "Kidney Cancer - Risk Factors and Prevention". Cancer.Net. 2012-06-25. Retrieved 2019-12-02.
- ↑ Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Ungprasert P, Kittanamongkolchai W, Erickson SB (9 September 2014). "The Risk of Kidney Cancer in Patients with Kidney Stones: A Systematic Review and Meta-analysis". QJM. 108 (3): 205–12. doi:10.1093/qjmed/hcu195. PMID 25208892.
- ↑ "Risks and causes of kidney cancer". 2017-08-30.
- ↑ Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P (2005-03-10). "Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies". International Journal of Cancer. 114 (1): 101–108. doi:10.1002/ijc.20618. ISSN 0020-7136. PMID 15523697. S2CID 10136386.
- ↑ Sánchez-Martín FM, Millán-Rodríguez F, Urdaneta-Pignalosa G, Rubio-Briones J, Villavicencio-Mavrich H (2008). "Small Renal Masses: Incidental Diagnosis, Clinical Symptoms, and Prognostic Factors". Advances in Urology. 2008: 310694. doi:10.1155/2008/310694. ISSN 1687-6369. PMC 2629071. PMID 19165347.
- ↑ Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Toni Choueiri, Costello BA, Derweesh IH, Fishman M, Gallagher TH (2017-06-01). "Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology". Journal of the National Comprehensive Cancer Network. 15 (6): 804–834. doi:10.6004/jnccn.2017.0100. ISSN 1540-1405. PMID 28596261.
- ↑ Silverman SG, Israel GM, Herts BR, Richie JP (October 2008). "Management of the incidental renal mass". Radiology. 249 (1): 16–31. doi:10.1148/radiol.2491070783. ISSN 1527-1315. PMID 18796665. S2CID 23327389.
- ↑ "Cancer | Definition of Cancer by Lexico". Lexico Dictionaries | English. Archived from the original on November 12, 2019. Retrieved 2019-11-12.
- ↑ Israel GM, Bosniak MA (2005). "How I Do It: Evaluating Renal Masses". Radiology. 236 (2): 441–450. doi:10.1148/radiol.2362040218. ISSN 0033-8419. PMID 16040900. S2CID 1916092.
- ↑ Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman SS, Wells SA (2019-06-18). "Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment". Radiology. 292 (2): 475–488. doi:10.1148/radiol.2019182646. ISSN 0033-8419. PMC 6677285. PMID 31210616.
- 1 2 3 4 5 6 7 8 9 10 "Renal Cancer: Renal Mass & Localized Renal Cancer Guideline - American Urological Association". www.auanet.org. Archived from the original on 2020-02-15. Retrieved 2019-10-29.
- 1 2 3 4 Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman M, Gallagher TH (2017-06-01). "Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology". Journal of the National Comprehensive Cancer Network. 15 (6): 804–834. doi:10.6004/jnccn.2017.0100. ISSN 1540-1405. PMID 28596261.
- ↑ Subramaniam RM, Wilson RF, Turban S, Suarez-Cuervo C, Zhang A, Sherrod C, Aboagye J, Eng J, Choi MJ (2016). Contrast-Induced Nephropathy: Comparative Effectiveness of Preventive Measures. AHRQ Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US). PMID 26866209.
- ↑ "Kidney Cancer | CDC". www.cdc.gov. 2019-07-09. Retrieved 2019-11-12.
- ↑ "Renal Cell Cancer Treatment (PDQ®)–Patient Version". National Cancer Institute. 2004-02-20. Retrieved 2019-11-12.
- 1 2 3 "Transitional Cell Cancer of the Renal Pelvis and Ureter Treatment (PDQ®)–Patient Version". National Cancer Institute. 2004-02-20. Retrieved 2019-11-12.
- ↑ Kuroda N, Katto K, Tamura M, Shiotsu T, Hes O, Michal M, Nagashima Y, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, Lee GH (January 2008). "Carcinoid tumor of the renal pelvis: consideration on the histogenesis". Pathol. Int. 58 (1): 51–4. doi:10.1111/j.1440-1827.2007.02188.x. PMID 18067641. S2CID 27645211.
- ↑ Chiu KC, Lin MC, Liang YC, Chen CY (2008). "Renal carcinosarcoma: case report and review of literature". Renal Failure. 30 (10): 1034–9. doi:10.1080/08860220802403192. PMID 19016157.
- ↑ Picozzi S, Casellato S, Bozzini G, Ratti D, Macchi A, Rubino B, Pace G, Carmignani L (November 2013). "Inverted papilloma of the bladder: a review and an analysis of the recent literature of 365 patients". Urologic Oncology. 31 (8): 1584–1590. doi:10.1016/j.urolonc.2012.03.009. ISSN 1873-2496. PMID 22520573.
- ↑ Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z (May 2006). "2004 WHO classification of the renal tumors of the adults". European Urology. 49 (5): 798–805. doi:10.1016/j.eururo.2005.11.035. ISSN 0302-2838. PMID 16442207.
- ↑ Thyavihally YB, Tongaonkar HB, Desai SB (September 2005). "Benign mixed epithelial stromal tumor of the renal pelvis with exophytic growth: case report". Int Semin Surg Oncol. 2: 18. doi:10.1186/1477-7800-2-18. PMC 1215508. PMID 16150156.
- ↑ Mantilla JG, Antic T, Tretiakova M (2017). "GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics". Hum Pathol. 66: 152–158. doi:10.1016/j.humpath.2017.06.016. PMID 28705707.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Behtash G. Nezami, M.D., Gregory MacLennan, M.D. "Kidney tumor - Adult renal cell carcinoma - common - Clear cell". Pathology Outlines.
{{cite web}}: CS1 maint: multiple names: authors list (link) Topic Completed: 20 April 2021. Minor changes: 20 April 2021 - 1 2 Motzer RJ, Jonasch E, Agarwal N, Bhayani S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman M, Gallagher TH (2017-06-01). "Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology". Journal of the National Comprehensive Cancer Network. 15 (6): 804–834. doi:10.6004/jnccn.2017.0100. ISSN 1540-1405. PMID 28596261.
- ↑ Patel HD, Johnson MH, Pierorazio PM, Sozio SM, Sharma R, Iyoha E, Bass EB, Allaf ME (May 2016). "Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature". The Journal of Urology. 195 (5): 1340–1347. doi:10.1016/j.juro.2015.11.029. ISSN 1527-3792. PMC 5609078. PMID 26901507.
- ↑ "Kidney Cancer Stages | Renal Cell Carcinoma Staging". www.cancer.org. Retrieved 2019-11-12.
- 1 2 3 4 AJCC cancer staging manual. Edge, Stephen B., American Joint Committee on Cancer. (7th ed.). New York: Springer. 2010. ISBN 9780387884400. OCLC 316431417.
{{cite book}}: CS1 maint: others (link) - ↑ "Kidney Cancer Stages". cancer.org. Last Revised: February 1, 2020
- ↑ Swami U, Nussenzveig RH, Haaland B, Agarwal N (2019). "Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement". Annals of Translational Medicine. 7 (S1): S18. doi:10.21037/atm.2019.01.50. ISSN 2305-5839. PMC 6462602. PMID 31032299.
- ↑ "Kidney Cancer (Adult) – Renal Cell Carcinoma" (PDF). American Cancer Society. Archived from the original (PDF) on 2017-02-22. Last Revised: May 16, 2016
- ↑ "Early stage and locally advanced kidney cancer treatment". 2017-08-30.
- ↑ "Advanced kidney cancer". 2017-08-30.
- ↑ Choueiri TK, et al. (2021-08-19). "Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma". New England Journal of Medicine. 385 (8): 683–694. doi:10.1056/nejmoa2106391. PMID 34407342. S2CID 237215136.
- ↑ "Renal cell carcinoma | HemOnc.org - A Hematology Oncology Wiki". hemonc.org.
- ↑ "National Comprehensive Cancer Network" (PDF).
- ↑ "Biological therapy for kidney cancer". 2017-08-30.
- ↑ Jonasch, Eric, Messner, Carolyn (August 2012). "CancerCare Connect – Treatment Update: Kidney Cancer" (PDF). Cancer Care, Inc. Archived from the original (PDF) on 2011-09-25. Retrieved 2012-08-29.
- 1 2 Syn NL, Teng MW, Mok TS, Soo RA (2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ↑ "FDA approves Opdivo to treat advanced form of kidney cancer". Food and Drug Administration.
- ↑ Philips GK, Atkins MB (2014). "New agents and new targets for renal cell carcinoma". American Society of Clinical Oncology Educational Book / ASCO. American Society of Clinical Oncology. Meeting. 34 (34): e222-7. doi:10.14694/EdBook_AM.2014.34.e222. PMID 24857106.
- ↑ "Drugs@FDA: FDA Approved Drug Products".
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208692s000lbl.pdf
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206947s000lbl.pdf
- 1 2 "Wilms' tumour (kidney cancer in children)". 2017-08-30. Archived from the original on 2012-04-06.
- ↑ Lindblad, P. and Adami H.O, Kidney Cancer, in Textbook of Cancer.
- ↑ GLOBOCAN 2002, Cancer Incidence, Mortality and Prevalence Worldwide 2002 estimates. 2006.
- ↑ Klaassen Z, Sayyid RK, Wallis CJ (2019). "Lessons Learned from the Global Epidemiology of Kidney Cancer: A Refresher in Epidemiology 101". European Urology. 75 (1): 85–87. doi:10.1016/j.eururo.2018.09.035. PMID 30274700. S2CID 52900491.
- ↑ "Cancer of the Kidney and Renal Pelvis – SEER Stat Fact Sheets". National Cancer Institute, U.S. National Institutes of Health. Retrieved 2013-02-07.
- ↑ Lynch CF, West MM, Davila JA, Platz CE (2007). "Chapter 24: Cancers of the Kidney and Renal Pelvic". In Ries L, Young JL, Keel GE, Eisner MP, Horner M (eds.). SEER Survival Monograph: Cancer Survival Among Adults: US SEER Program, 1988–2001, Patient and Tumor Characteristics. Vol. NIH Pub. No. 07-6215. Bethesda, MD: National Cancer Institute. pp. 193–202. Archived from the original on 2013-10-10.
- ↑ Ferlay J, et al. (2007). "Estimates of the cancer incidence and mortality in Europe in 2006". Annals of Oncology. 18 (3): 581–92. doi:10.1093/annonc/mdl498. PMID 17287242.
- ↑ "Kidney cancer statistics". Cancer Research UK. Retrieved 27 October 2014.