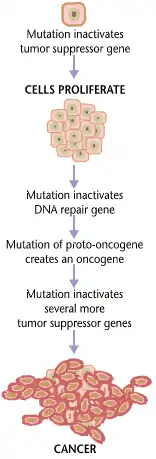
Cancer is caused by genetic changes leading to uncontrolled cell growth and tumor formation. The basic cause of sporadic (non-familial) cancers is DNA damage and genomic instability.[1][2] A minority of cancers are due to inherited genetic mutations.[3] Most cancers are related to environmental, lifestyle, or behavioral exposures.[4] Cancer is generally not contagious in humans, though it can be caused by oncoviruses and cancer bacteria. The term "environmental", as used by cancer researchers, refers to everything outside the body that interacts with humans.[5] The environment is not limited to the biophysical environment (e.g. exposure to factors such as air pollution or sunlight), but also includes lifestyle and behavioral factors.[6]
Over one third of cancer deaths worldwide (and about 75–80% in the United States) are potentially avoidable by reducing exposure to known factors.[7][8] Common environmental factors that contribute to cancer death include exposure to different chemical and physical agents (tobacco use accounts for 25–30% of cancer deaths), environmental pollutants, diet and obesity (30–35%), infections (15–20%), and radiation (both ionizing and non-ionizing, up to 10%).[9] These factors act, at least partly, by altering the function of genes within cells.[10] Typically many such genetic changes are required before cancer develops.[10] Aging has been repeatedly and consistently regarded as an important aspect to consider when evaluating the risk factors for the development of particular cancers. Many molecular and cellular changes involved in the development of cancer accumulate during the aging process and eventually manifest as cancer.[11]
Genetics
Although there are over 50 identifiable hereditary forms of cancer, less than 0.3% of the population are carriers of a cancer-related genetic mutation and these make up less than 3–10% of all cancer cases.[3] The vast majority of cancers are non-hereditary ("sporadic cancers"). Hereditary cancers are primarily caused by an inherited genetic defect. A cancer syndrome or family cancer syndrome is a genetic disorder in which inherited genetic mutations in one or more genes predisposes the affected individuals to the development of cancers and may also cause the early onset of these cancers. Although cancer syndromes exhibit an increased risk of cancer, the risk varies. For some of these diseases, cancer is not the primary feature and is a rare consequence.
Many of the cancer syndrome cases are caused by mutations in tumor suppressor genes that regulate cell growth. Other common mutations alter the function of DNA repair genes, oncogenes and genes involved in the production of blood vessels.[12] Certain inherited mutations in the genes BRCA1 and BRCA2 with a more than 75% risk of breast cancer and ovarian cancer.[3] Some of the inherited genetic disorders that can cause colorectal cancer include familial adenomatous polyposis and hereditary non-polyposis colon cancer; however, these represent less than 5% of colon cancer cases.[13] In many cases, genetic testing can be used to identify mutated genes or chromosomes that are passed through generations.
Gene mutations are classified as germline or somatic depending on the cell type where they appear (germline cells include the egg and the sperm and somatic cells are those forming the body). The germline mutations are carried through generations and increase the risk of cancer.
Cancer syndromes
Physical and chemical agents
Particular substances, known as carcinogens, have been linked to specific types of cancer. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise in both natural and synthetic substances.[14] It is estimated that approximately 20,000 cancer deaths and 40,000 new cases of cancer each year in the U.S. are attributable to occupation.[15] Every year, at least 200,000 people die worldwide from cancer related to their workplace.[16] Millions of workers run the risk of developing cancers such as lung cancer and mesothelioma from inhaling asbestos fibers and tobacco smoke, or leukemia from exposure to benzene at their workplaces.[16] Cancer related to one's occupation is believed to represent between 2–20% of all cases.[17] Most cancer deaths caused by occupational risk factors occur in the developed world.[16] Job stress does not appear to be a significant factor at least in lung, colorectal, breast and prostate cancers.[18] Participation in Operation Ranchhand in Vietnam during the Vietnam war, or living near a golf course, or living on a farm would increase the risk of non-Hodgkins lymphoma due to exposure to the chemical 2,4-D. When 2,4-D is mixed with another chemical pesticide or herbicide, 2,4-T, at a 50:50 ratio, they are collectively known as Agent Orange.
Smoking


Tobacco smoking is associated with many forms of cancer,[20] and causes 80% of lung cancer.[21] Decades of research has demonstrated the link between tobacco use and cancer in the lung, larynx, head, neck, stomach, bladder, kidney, esophagus and pancreas.[22] There is some evidence suggesting a small increased risk of developing myeloid leukemia, squamous cell sinonasal cancer, liver cancer, colorectal cancer, cancers of the gallbladder, the adrenal gland, the small intestine, and various childhood cancers.[22] Seven toxicants in cigarette smoke have been identified that are most associated with respiratory tract carcinogenesis.[23] The mechanism of action of two of them, acrylonitrile and acrolein, appears to involve oxidative stress and oxidative DNA damage.[24][25] The other five toxicants, acetaldehyde, cadmium, ethylene oxide, formaldehyde and isoprene act through various mechanisms including direct interaction with DNA. Tobacco smoke contains over fifty known carcinogens, including nitrosamines and polycyclic aromatic hydrocarbons.[26] Tobacco is responsible for about one in three of all cancer deaths in the developed world,[20] and about one in five worldwide.[26] Lung cancer death rates in the United States have mirrored smoking patterns, with increases in smoking followed by dramatic increases in lung cancer death rates and, more recently, decreases in smoking rates since the 1950s followed by decreases in lung cancer death rates in men since 1990.[27][28] However, the numbers of smokers worldwide is still rising, leading to what some organizations have described as the tobacco epidemic.[29]
Electronic cigarettes or e-cigarettes are handheld electronic devices that simulate the feeling of tobacco smoking. Daily long-term use of high voltage (5.0 V) electronic cigarettes may generate formaldehyde-forming chemicals at a greater level than smoking, which was determined to be a lifetime cancer risk of approximately 5 to 15 times greater than smoking.[30] However, the overall safety and long-term health effects of electronic cigarettes is still uncertain.[31]
Materials
Some substances cause cancer primarily through their physical, rather than chemical, effects on cells.[32] A prominent example of this is prolonged exposure to asbestos, naturally occurring mineral fibers which are a major cause of mesothelioma, which is a cancer of the serous membrane, usually the serous membrane surrounding the lungs.[32] Other substances in this category, including both naturally occurring and synthetic asbestos-like fibers such as wollastonite, attapulgite, glass wool, and rock wool, are believed to have similar effects.[32] Non-fibrous particulate materials that cause cancer include powdered metallic cobalt and nickel, and crystalline silica (quartz, cristobalite, and tridymite).[32] Usually, physical carcinogens must get inside the body (such as through inhaling tiny pieces) and require years of exposure to develop cancer.[32] Common occupational carcinogens include:[33]
Lifestyle
Many different lifestyle factors contribute to increasing cancer risk. Together, diet and obesity are related to approximately 30–35% of cancer deaths.[9][34] Dietary recommendations for cancer prevention typically include an emphasis on vegetables, fruit, whole grains, and fish, and avoidance of processed meat, red meat, animal fats, and refined carbohydrates.[35] The evidence to support these dietary changes is not definitive.[36]
Alcohol
Alcohol is an example of a chemical carcinogen. The World Health Organization has classified alcohol as a Group 1 carcinogen.[37] In Western Europe 10% of cancers in males and 3% of cancers in females are attributed to alcohol.[38] Worldwide, 3.6% of all cancer cases and 3.5% of cancer deaths are attributable to alcohol.[39] In particular, alcohol use has been shown to increase the risk of developing cancers of the mouth, esophagus, pharynx, larynx, stomach, liver, ovaries, and colon.[40] The main mechanism of cancer development involves increased exposure to acetaldehyde, a carcinogen and breakdown product of ethanol.[41] Acetaldehyde induces DNA interstrand crosslinks, a form of DNA damage. These can be repaired by an inaccurate replication-coupled DNA repair pathway.[42] This repair pathway results in increased mutation frequency and altered mutational spectrum.[42] Other mechanisms have been proposed, including alcohol-related nutritional deficiencies, changes in DNA methylation, and induction of oxidative stress in tissues.[43]
Diet
Some specific foods have been linked to specific cancers. Studies have shown that individuals that eat red or processed meat have a higher risk of developing breast cancer, prostate cancer, and pancreatic cancer.[44] This may be partially explained by the presence of carcinogens in food cooked at high temperatures.[45] Several risk factors for the development of colorectal cancer include high intake of fat, alcohol, red and processed meats, obesity, and lack of physical exercise.[46] A high-salt diet is linked to gastric cancer. Aflatoxin B1, a frequent food contaminate, is associated with liver cancer. Betel nut chewing has been shown to cause oral cancers.[47]
The relationship between diet and the development of particular cancers may partly explain differences in cancer incidence in different countries. For example, gastric cancer is more common in Japan due to the frequency of high-salt diets and colon cancer is more common in the United States due to the increased intake of processed and red meats.[48] Immigrant communities tend to develop the cancer risk profile of their new country, often within one to two generations, suggesting a substantial link between diet and cancer.[49][50]
When deoxycholate was added to the food of mice so that their feces contained deoxycholate at about the same level present in feces of human on a high fat diet, 45% to 56% of the mice developed colon cancer over the next 10 months, while none of the mice on a diet without deoxycholate developed cancer.[51][52] A recent prospective human study investigating the relationship between microbial metabolites and cancer found a strong correlation between circulating deoxycholate as well as other specific bile acids and colorectal cancer risk in women.[53]
Obesity
In the United States, excess body weight is associated with the development of many types of cancer and is a factor in 14–20% of all cancer deaths.[34] Every year, nearly 85,000 new cancer diagnoses in the United States are related to obesity.[54] Individuals who underwent bariatric surgery for weight loss have reduced cancer incidence and mortality.[54]
There is an association between obesity and colon cancer, post-menopausal breast cancer, endometrial cancer, kidney cancer, and esophageal cancer.[54] Obesity has also been linked with the development of liver cancer.[55] The current understanding regarding the mechanism of cancer development in obesity relates to abnormal levels of metabolic proteins (including insulin-like growth factors) and sex hormones (estrogens, androgens and progestogens).[54] Adipose tissue also creates an inflammatory environment which may contribute to the development of cancers.[56] Adipose tissue dysregulation can result in oxidative stress leading to oxidative DNA damage and cancer associated genetic instability.[57]
Physical inactivity is believed to contribute to cancer risk not only through its effect on body weight but also through negative effects on immune system and endocrine system.[34] More than half of the effect from diet is due to overnutrition rather than from eating too little healthy foods.[54]
Hormones

Some hormones play a role in the development of cancer by promoting cell proliferation.[58] Insulin-like growth factors and their binding proteins play a key role in cancer cell growth, differentiation and apoptosis, suggesting possible involvement in carcinogenesis.[59]
Hormones are important agents in sex-related cancers such as cancer of the breast, endometrium, prostate, ovary, and testis, and also of thyroid cancer and bone cancer.[58] For example, the daughters of women who have breast cancer have significantly higher levels of estrogen and progesterone than the daughters of women without breast cancer. These higher hormone levels may explain why these women have higher risk of breast cancer, even in the absence of a breast-cancer gene.[58] Similarly, men of African ancestry have significantly higher levels of testosterone than men of European ancestry, and have a correspondingly much higher level of prostate cancer.[58] Men of Asian ancestry, with the lowest levels of testosterone-activating androstanediol glucuronide, have the lowest levels of prostate cancer.[58]
Other factors are also relevant: obese people have higher levels of some hormones associated with cancer and a higher rate of those cancers.[58] Women who take hormone replacement therapy have a higher risk of developing cancers associated with those hormones.[58] On the other hand, people who exercise far more than average have lower levels of these hormones, and lower risk of cancer.[58] Osteosarcoma may be promoted by growth hormones.[58]
Some treatments and prevention approaches leverage this cause by artificially reducing hormone levels, and thus discouraging hormone-sensitive cancers. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing or even undergo cell death.[58] Perhaps the most familiar example of hormonal therapy in oncology is the use of the selective estrogen-receptor modulator tamoxifen for the treatment of breast cancer. Another class of hormonal agents, aromatase inhibitors, now have an expanding role in the treatment of breast cancer.
Infection and inflammation
Worldwide, approximately 18% of cancer cases are related to infectious diseases.[9][60] This proportion varies in different regions of the world from a high of 25% in Africa to less than 10% in the developed world.[9] Viruses are the usual infectious agents that cause cancer but bacteria and parasites also contribute. Infectious organisms that increase the risk of cancer are frequently a source of DNA damage or genomic instability.
Viruses
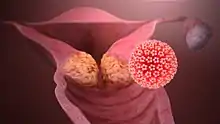
Viral infection is a major risk factor for cervical and liver cancer.[61] A virus that can cause cancer is called an oncovirus. These include human papillomavirus (cervical carcinoma), Epstein–Barr virus (B-cell lymphoproliferative disease and nasopharyngeal carcinoma), Kaposi's sarcoma herpesvirus (Kaposi's sarcoma and primary effusion lymphomas), hepatitis B and hepatitis C viruses (hepatocellular carcinoma), and Human T-cell leukemia virus-1 (T-cell leukemias).
In Western developed countries, human papillomavirus (HPV), hepatitis B virus (HBV) and hepatitis C virus (HCV) are the most common oncoviruses.[62] In the United States, HPV causes most cervical cancers, as well as some cancers of the vagina, vulva, penis, anus, rectum, throat, tongue and tonsils.[63] Among high-risk HPV viruses, the HPV E6 and E7 oncoproteins inactivate tumor suppressor genes when infecting cells. In addition, the oncoproteins independently induce genomic instability in normal human cells, leading to an increased risk of cancer development.[64] Individuals with chronic hepatitis B virus infection are more than 200 times more likely to develop liver cancer than uninfected individuals.[65] Liver cirrhosis, whether from chronic viral hepatitis infection or excessive alcohol use, is independently associated with the development of liver cancer, but the combination of cirrhosis and viral hepatitis presents the highest risk of liver cancer development.[65]
Bacteria and parasites
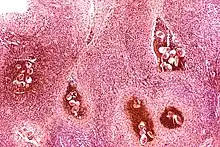
Certain bacterial infections also increase the risk of cancer, as seen in Helicobacter pylori-induced gastric carcinoma.[66] The mechanism by which H. pylori causes cancer may involve chronic inflammation or the direct action of some of the bacteria's virulence factors.[67] Parasitic infections strongly associated with cancer include Schistosoma haematobium (squamous cell carcinoma of the bladder) and the liver flukes, Opisthorchis viverrini and Clonorchis sinensis (cholangiocarcinoma).[68] Inflammation triggered by the worm's eggs appears to be the cancer-causing mechanism. Certain parasitic infections can also increase the presence of carcinogenic compounds in the body, leading to the development of cancers.[69] Tuberculosis infection, caused by the mycobacterium M. tuberculosis, has also been linked with the development of lung cancer.[70]
Inflammation
There is evidence that inflammation itself plays an important role in the development and progression of cancer.[71] Chronic inflammation can lead to DNA damage over time and the accumulation of random genetic alterations in cancer cells.[72] Inflammation can contribute to proliferation, survival, angiogenesis and migration of cancer cells by influencing tumor microenvironment.[73] Individuals with inflammatory bowel disease are at increased risk of developing colorectal cancers.[13]
Radiation
Up to 10% of invasive cancers are related to radiation exposure, including both non-ionizing radiation and ionizing radiation.[9] Unlike chemical or physical triggers for cancer, ionizing radiation hits molecules within cells randomly. If it happens to strike a chromosome, it can break the chromosome, result in an abnormal number of chromosomes, inactivate one or more genes in the part of the chromosome that it hit, delete parts of the DNA sequence, cause chromosome translocations, or cause other types of chromosome abnormalities.[74] Major damage normally results in the cell dying, but smaller damage may leave a stable, partly functional cell that may be capable of proliferating and developing into cancer, especially if tumor suppressor genes were damaged by the radiation.[74] Three independent stages appear to be involved in the creation of cancer with ionizing radiation: morphological changes to the cell, acquiring cellular immortality (losing normal, life-limiting cell regulatory processes), and adaptations that favor formation of a tumor.[74] Even if the radiation particle does not strike the DNA directly, it triggers responses from cells that indirectly increase the likelihood of mutations.[74]
Non-ionizing radiation
.jpg.webp)
Not all types of electromagnetic radiation are carcinogenic. Low-energy waves on the electromagnetic spectrum including radio waves, microwaves, infrared radiation and visible light are thought not to be because they have insufficient energy to break chemical bonds. Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been described as a possible carcinogen by the World Health Organization's International Agency for Research on Cancer.[75][76] However, studies have not found a consistent link between cell phone radiation and cancer risk.[77]
Higher-energy radiation, including ultraviolet radiation (present in sunlight), x-rays, and gamma radiation, generally is carcinogenic, if received in sufficient doses. Prolonged exposure to ultraviolet radiation from the sun can lead to melanoma and other skin malignancies.[78] The vast majority of non-invasive cancers are non-melanoma skin cancers caused by non-ionizing ultraviolet radiation. Clear evidence establishes ultraviolet radiation, especially the non-ionizing medium wave UVB, as the cause of most non-melanoma skin cancers, which are the most common forms of cancer in the world.[78]
Ionizing radiation
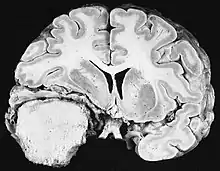
Sources of ionizing radiation include medical imaging, and radon gas. Ionizing radiation is not a particularly strong mutagen.[74] Medical use of ionizing radiation is a growing source of radiation-induced cancers. Ionizing radiation may be used to treat other cancers, but this may, in some cases, induce a second form of cancer.[74] Radiation can cause cancer in most parts of the body, in all animals, and at any age, although radiation-induced solid tumors usually take 10–15 years, and can take up to 40 years, to become clinically manifest, and radiation-induced leukemias typically require 2–10 years to appear.[74] Radiation-induced meningiomas are an uncommon complication of cranial irradiation.[79] Some people, such as those with nevoid basal cell carcinoma syndrome or retinoblastoma, are more susceptible than average to developing cancer from radiation exposure.[74] Children and adolescents are twice as likely to develop radiation-induced leukemia as adults; radiation exposure before birth has ten times the effect.[74]
Ionizing radiation is also used in some kinds of medical imaging. In industrialized countries, medical imaging contributes almost as much radiation dose to the public as natural background radiation. Nuclear medicine techniques involve the injection of radioactive pharmaceuticals directly into the bloodstream. Radiotherapy deliberately deliver high doses of radiation to tumors and surrounding tissues as a form of disease treatment. It is estimated that 0.4% of cancers in 2007 in the United States are due to CTs performed in the past and that this may increase to as high as 1.5–2% with rates of CT usage during this same time period.[80]
Residential exposure to radon gas has similar cancer risks as passive smoking.[74] Low-dose exposures, such as living near a nuclear power plant, are generally believed to have no or very little effect on cancer development.[74] Radiation is a more potent source of cancer when it is combined with other cancer-causing agents, such as radon gas exposure plus smoking tobacco.[74]
Rare causes
Organ transplantation
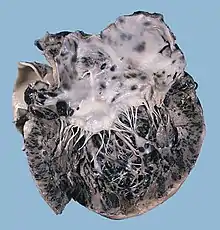
The development of donor-derived tumors from organ transplants is exceedingly rare. The main cause of organ transplant associated tumors seems to be malignant melanoma, that was undetected at the time of organ harvest.[81] There have also been reports of Kaposi's sarcoma occurring after transplantation due to tumorous outgrowth of virus-infected donor cells.[82]
Trauma
Physical trauma resulting in cancer is relatively rare.[83] Claims that breaking bones resulted in bone cancer, for example, have never been proven.[83] Similarly, physical trauma is not accepted as a cause for cervical cancer, breast cancer, or brain cancer.[83] One accepted source is frequent, long-term application of hot objects to the body. It is possible that repeated burns on the same part of the body, such as those produced by kanger and kairo heaters (charcoal hand warmers), may produce skin cancer, especially if carcinogenic chemicals are also present.[83]
Frequently drinking scalding hot tea may produce esophageal cancer.[83] Generally, it is believed that the cancer arises, or a pre-existing cancer is encouraged, during the process of repairing the trauma, rather than the cancer being caused directly by the trauma.[83] However, repeated injuries to the same tissues might promote excessive cell proliferation, which could then increase the odds of a cancerous mutation.
Maternal-fetal transmission
In the United States, approximately 3,500 pregnant women have a malignancy annually, and transplacental transmission of acute leukemia, lymphoma, melanoma and carcinoma from mother to fetus has been observed.[84] Excepting the rare transmissions that occur with pregnancies and only a marginal few organ donors, cancer is generally not a transmissible disease. The main reason for this is tissue graft rejection caused by MHC incompatibility.[84] In humans and other vertebrates, the immune system uses MHC antigens to differentiate between "self" and "non-self" cells because these antigens are different from person to person. When non-self antigens are encountered, the immune system reacts against the appropriate cell. Such reactions may protect against tumor cell engraftment by eliminating implanted cells.
References
- ↑ Basu, Ashis K (23 March 2018). "DNA Damage, Mutagenesis and Cancer". Int. J. Mol. Sci. (Review). 19 (4): 970. doi:10.3390/ijms19040970. PMC 5979367. PMID 29570697.
- ↑ Ferguson LR, Chen H, Collins AR, Connell M, Damia G, Dasgupta S, et al. (December 2015). "Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition". Seminars in Cancer Biology (Review). Elsevier. 35 Suppl (Suppl): S5–S24. doi:10.1016/j.semcancer.2015.03.005. PMC 4600419. PMID 25869442.
- 1 2 3 Roukos DH (April 2009). "Genome-wide association studies: how predictable is a person's cancer risk?". Expert Review of Anticancer Therapy (Editorial). 9 (4): 389–92. doi:10.1586/era.09.12. PMID 19374592. S2CID 24746283.

- ↑ Stewart BW, Wild CP, eds. (2014). "Cancer etiology". World Cancer Report 2014. World Health Organization. pp. 16–54. ISBN 978-9283204299.
- ↑ Cancer and the Environment: What you Need to Know, What You Can Do. NIH Publication No. 03-2039: National Institutes of Health. 2003.
Cancer develops over several years and has many causes. Several factors both inside and outside the body contribute to the development of cancer. In this context, scientists refer to everything outside the body that interacts with humans as 'environmental'.
{{cite book}}: CS1 maint: location (link) - ↑ Manton KG, Akushevich I, Kravchenko J (2009). "3 Cancer Risk Factors (3.2 Environmental Cancer Risk Factors)". Cancer mortality and morbidity patterns from the U. S. population: an interdisciplinary approach. Berlin: Springer. p. 118. ISBN 978-0-387-78192-1 – via Open Library.
The term environment refers not only to air, water, and soil but also to substances and conditions at home and at the workplace, including diet, smoking, alcohol, drugs, exposure to chemicals, sunlight, ionizing radiation, electromagnetic fields, infectious agents, etc. Lifestyle, economic and behavioral factors are all aspects of our environment.
- ↑ Doll R, Peto R (June 1981). "The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today". Journal of the National Cancer Institute. 66 (6): 1191–308. doi:10.1093/jnci/66.6.1192. PMID 7017215.
- ↑ Whiteman DC, Wilson LF (October 2016). "The fractions of cancer attributable to modifiable factors: A global review". Cancer Epidemiology (Review). Elsevier. 44: 203–221. doi:10.1016/j.canep.2016.06.013. ISSN 1877-783X. PMID 27460784 – via ScienceDirect.
- 1 2 3 4 5 Anand P, Kunnumakkara AB, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (September 2008). "Cancer is a preventable disease that requires major lifestyle changes". Pharmaceutical Research (Expert review). 25 (9): 2097–116. doi:10.1007/s11095-008-9661-9. PMC 2515569. PMID 18626751.
- 1 2 World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 1.1. ISBN 978-9283204299.
- ↑ "Cancer Fact sheet N°297". World Health Organization. February 2014. Retrieved 10 June 2014.
- ↑ Hodgson S (January 2008). "Mechanisms of inherited cancer susceptibility". Journal of Zhejiang University Science B. 9 (1): 1–4. doi:10.1631/jzus.B073001. PMC 2170461. PMID 18196605.
- 1 2 World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.5. ISBN 978-9283204299.
- ↑ Ames, Bruce N.; Gold, Lois Swirsky (17 January 2000). "Paracelsus to parascience: the environmental cancer distraction". Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 447 (1): 3–13. doi:10.1016/S0027-5107(99)00194-3. ISSN 0027-5107. PMID 10686303.
- ↑ "National Institute for Occupational Safety and Health- Occupational Cancer". United States National Institute for Occupational Safety and Health. Retrieved 13 October 2007.
- 1 2 3 "WHO calls for prevention of cancer through healthy workplaces" (Press release). World Health Organization. 27 April 2007. Archived from the original on 1 May 2007. Retrieved 13 October 2007.
- ↑ Irigaray P, Newby JA, Clapp R, Hardell L, Howard V, Montagnier L, Epstein S, Belpomme D (December 2007). "Lifestyle-related factors and environmental agents causing cancer: an overview". Biomedicine & Pharmacotherapy. 61 (10): 640–58. doi:10.1016/j.biopha.2007.10.006. PMID 18055160.
- ↑ Heikkilä K, Nyberg ST, Theorell T, Fransson EI, Alfredsson L, Bjorner JB, et al. (February 2013). "Work stress and risk of cancer: meta-analysis of 5700 incident cancer events in 116,000 European men and women". BMJ. 346: f165. doi:10.1136/bmj.f165. PMC 3567204. PMID 23393080.
- ↑ "Share of cancer deaths attributed to tobacco". Our World in Data. Retrieved 5 March 2020.
- 1 2 Sasco AJ, Secretan MB, Straif K (August 2004). "Tobacco smoking and cancer: a brief review of recent epidemiological evidence". Lung Cancer. 45 (Suppl 2): S3–9. doi:10.1016/j.lungcan.2004.07.998. PMID 15552776.
- ↑ Biesalski HK, Bueno de Mesquita B, Chesson A, Chytil F, Grimble R, Hermus RJ, Köhrle J, Lotan R, Norpoth K, Pastorino U, Thurnham D (1998). "European Consensus Statement on Lung Cancer: risk factors and prevention. Lung Cancer Panel". CA: A Cancer Journal for Clinicians. 48 (3): 167–76, discussion 164–6. doi:10.3322/canjclin.48.3.167. PMID 9594919. S2CID 20891885.
- 1 2 Kuper H, Boffetta P, Adami HO (September 2002). "Tobacco use and cancer causation: association by tumour type". Journal of Internal Medicine. 252 (3): 206–24. doi:10.1046/j.1365-2796.2002.01022.x. PMID 12270001. S2CID 6132726.
- ↑ Cunningham FH, Fiebelkorn S, Johnson M, Meredith C. A novel application of the Margin of Exposure approach: segregation of tobacco smoke toxicants. Food Chem Toxicol. 2011 Nov;49(11):2921-33. doi: 10.1016/j.fct.2011.07.019. Epub 2011 Jul 23. PMID 21802474
- ↑ Pu X, Kamendulis LM, Klaunig JE. Acrylonitrile-induced oxidative stress and oxidative DNA damage in male Sprague-Dawley rats. Toxicol Sci. 2009;111(1):64-71. doi:10.1093/toxsci/kfp133
- ↑ Li L, Jiang L, Geng C, Cao J, Zhong L. The role of oxidative stress in acrolein-induced DNA damage in HepG2 cells. Free Radic Res. 2008 Apr;42(4):354-61. doi: 10.1080/10715760802008114. PMID 18404534
- 1 2 Kuper H, Adami HO, Boffetta P (June 2002). "Tobacco use, cancer causation and public health impact". Journal of Internal Medicine. 251 (6): 455–66. doi:10.1046/j.1365-2796.2002.00993.x. PMID 12028500. S2CID 9172672.
- ↑ Thun MJ, Jemal A (October 2006). "How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking?". Tobacco Control. 15 (5): 345–7. doi:10.1136/tc.2006.017749. PMC 2563648. PMID 16998161.
- ↑ Dubey S, Powell CA (May 2008). "Update in lung cancer 2007". American Journal of Respiratory and Critical Care Medicine. 177 (9): 941–6. doi:10.1164/rccm.200801-107UP. PMC 2720127. PMID 18434333.
- ↑ Proctor RN (May 2004). "The global smoking epidemic: a history and status report". Clinical Lung Cancer. 5 (6): 371–6. doi:10.3816/CLC.2004.n.016. PMID 15217537.
- ↑ Cooke A, Fergeson J, Bulkhi A, Casale TB (2015). "The Electronic Cigarette: The Good, the Bad, and the Ugly". The Journal of Allergy and Clinical Immunology. In Practice. 3 (4): 498–505. doi:10.1016/j.jaip.2015.05.022. PMID 26164573.
- ↑ Ebbert, Jon O.; Agunwamba, Amenah A.; Rutten, Lila J. (January 2015). "Counseling patients on the use of electronic cigarettes". Mayo Clinic Proceedings. 90 (1): 128–134. doi:10.1016/j.mayocp.2014.11.004. ISSN 1942-5546. PMID 25572196.
- 1 2 3 4 5 Maltoni CF, Holland JF (2000). "Chapter 16: Physical Carcinogens". In Bast RC, Kufe DW, Pollock RE, et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 978-1-55009-113-7. Retrieved 31 January 2011.
- ↑ Robbins basic pathology. Kumar, Vinay, 1944-, Robbins, Stanley L. (Stanley Leonard), 1915-2003. (8th ed.). Philadelphia, PA: Saunders/Elsevier. 2007. pp. Table 6–2. ISBN 978-1416029731. OCLC 69672074.
{{cite book}}: CS1 maint: others (link) - 1 2 3 Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ (2006). "American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA: A Cancer Journal for Clinicians. 56 (5): 254–81, quiz 313–4. doi:10.3322/canjclin.56.5.254. PMID 17005596. S2CID 19823935.
- ↑ Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, Patel AV, Andrews K, Gansler T (January 2012). "American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity". CA: A Cancer Journal for Clinicians. 62 (1): 30–67. doi:10.3322/caac.20140. PMID 22237782. S2CID 2067308.
- ↑ Wicki A, Hagmann J (2011). "Diet and cancer". Swiss Medical Weekly. 141: w13250. doi:10.4414/smw.2011.13250. PMID 21904992.
- ↑ "IARC: IARC Strengthens its Findings on Several Carcinogenic Personal Habits and Household Exposures" (PDF). International Agency for Research on Cancer - World Health Organization. 2009.
- ↑ Schütze M, Boeing H, Pischon T, Rehm J, Kehoe T, Gmel G, et al. (April 2011). "Alcohol attributable burden of incidence of cancer in eight European countries based on results from prospective cohort study". BMJ. 342: d1584. doi:10.1136/bmj.d1584. PMC 3072472. PMID 21474525.
- ↑ Boffetta (August 2006). "The burden of cancer attributable to alcohol drinking". International Journal of Cancer. 119 (4): 884–7. doi:10.1002/ijc.21903. hdl:2434/22728. PMID 16557583. S2CID 14938863.
- ↑ "Alcohol Consumption and the Risk of Cancer". pubs.niaaa.nih.gov. Archived from the original on 21 February 2018. Retrieved 22 March 2018.
- ↑ Theruvathu JA, Jaruga P, Nath RG, Dizdaroglu M, Brooks PJ (2005). "Polyamines stimulate the formation of mutagenic 1,N2-propanodeoxyguanosine adducts from acetaldehyde". Nucleic Acids Research. 33 (11): 3513–20. doi:10.1093/nar/gki661. PMC 1156964. PMID 15972793.
- 1 2 Hodskinson MR, Bolner A, Sato K, Kamimae-Lanning AN, Rooijers K, Witte M, Mahesh M, Silhan J, Petek M, Williams DM, Kind J, Chin JW, Patel KJ, Knipscheer P. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms. Nature. 2020 Mar;579(7800):603-608. doi: 10.1038/s41586-020-2059-5. Epub 2020 Mar 4. PMID 32132710; PMCID: PMC7116288.
- ↑ Poschl G (May 2004). "Alcohol and Cancer". Alcohol and Alcoholism. 39 (3): 155–165. doi:10.1093/alcalc/agh057. PMID 15082451.
- ↑ Stewart B (2014). World Cancer Report 2014. World Health Organization. pp. 124–33. ISBN 9789283204299.
- ↑ Ferguson LR (February 2010). "Meat and cancer". Meat Science. 84 (2): 308–313. doi:10.1016/j.meatsci.2009.06.032. PMID 20374790.
- ↑ "Colorectal Cancer 2011 Report: Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer" (PDF). World Cancer Research Fund & American Institute for Cancer Research. 2011. Archived from the original (PDF) on 22 May 2019. Retrieved 22 March 2018.
- ↑ Park S, Bae J, Nam BH, Yoo KY (2008). "Aetiology of cancer in Asia". Asian Pacific Journal of Cancer Prevention. 9 (3): 371–80. PMID 18990005. Archived from the original (PDF) on 4 September 2011. Retrieved 17 July 2014.
- ↑ Brenner H, Rothenbacher D, Arndt V (2009). "Epidemiology of Stomach Cancer". Cancer Epidemiology. Methods in Molecular Biology. Vol. 472. pp. 467–77. doi:10.1007/978-1-60327-492-0_23. ISBN 978-1-60327-491-3. PMC 2166976. PMID 19107449.
- ↑ Buell P, Dunn JE (May 1965). "Cancer Mortality among Japanese Issei and Nisei of California". Cancer. 18 (5): 656–64. doi:10.1002/1097-0142(196505)18:5<656::AID-CNCR2820180515>3.0.CO;2-3. PMID 14278899. S2CID 39881987.
- ↑ Parkin, D. M.; Khlat, M. (May 1996). "Studies of cancer in migrants: rationale and methodology". European Journal of Cancer. 32A (5): 761–771. doi:10.1016/0959-8049(96)00062-7. ISSN 0959-8049. PMID 9081351.
- ↑ Prasad AR, Prasad S, Nguyen H, Facista A, Lewis C, Zaitlin B, et al. (July 2014). "Novel diet-related mouse model of colon cancer parallels human colon cancer". World Journal of Gastrointestinal Oncology. 6 (7): 225–43. doi:10.4251/wjgo.v6.i7.225. PMC 4092339. PMID 25024814
- ↑ Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H (August 2011). "Carcinogenicity of deoxycholate, a secondary bile acid". Archives of Toxicology. 85 (8): 863–71. doi:10.1007/s00204-011-0648-7. PMC 3149672. PMID 21267546.
- ↑ Erikka Loftfield, PhD, MPH, Roni T Falk, MS, Joshua N Sampson, PhD, Wen-Yi Huang, PhD, Autumn Hullings, MPH, Gwen Murphy, PhD, MPH, Stephanie J Weinstein, PhD, Demetrius Albanes, MD, Neal D Freedman, PhD, MPH, Rashmi Sinha, PhD, Prospective Associations of Circulating Bile Acids and Short-Chain Fatty Acids with Incident Colorectal Cancer, JNCI Cancer Spectrum, 2022;, pkac027, https://doi.org/10.1093/jncics/pkac027
- 1 2 3 4 5 6 Basen-Engquist K, Chang M (February 2011). "Obesity and cancer risk: recent review and evidence". Current Oncology Reports. 13 (1): 71–6. doi:10.1007/s11912-010-0139-7. PMC 3786180. PMID 21080117.
- ↑ Alzahrani B, Iseli TJ, Hebbard LW (April 2014). "Non-viral causes of liver cancer: does obesity led inflammation play a role?". Cancer Letters. 345 (2): 223–9. doi:10.1016/j.canlet.2013.08.036. PMID 24007864.
- ↑ Gilbert CA, Slingerland JM (14 January 2013). "Cytokines, obesity, and cancer: new insights on mechanisms linking obesity to cancer risk and progression". Annual Review of Medicine. 64 (1): 45–57. doi:10.1146/annurev-med-121211-091527. PMID 23121183.
- ↑ Kompella P, Vasquez KM. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Mol Carcinog. 2019 Sep;58(9):1531-1550. doi: 10.1002/mc.23048. Epub 2019 Jun 5. PMID 31168912; PMCID: PMC6692207
- 1 2 3 4 5 6 7 8 9 10 Henderson BE, Bernstein L, Ross RK (2000). "Chapter 13: Hormones and the Etiology of Cancer". In Bast RC, Kufe DW, Pollock RE, et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 978-1-55009-113-7. Retrieved 27 January 2011.
- ↑ Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM (May 2009). "Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis". International Journal of Cancer. 124 (10): 2416–29. doi:10.1002/ijc.24202. PMC 2743036. PMID 19142965.
- ↑ De Martel, Catherine; Ferlay, Jacques; Franceschi, Silvia; Vignat, Jérôme; Bray, Freddie; Forman, David; Plummer, Martyn (1 June 2012). "Global burden of cancers attributable to infections in 2008: a review and synthetic analysis". The Lancet Oncology. 13 (6): 607–615. doi:10.1016/S1470-2045(12)70137-7. ISSN 1470-2045. PMID 22575588.
- ↑ De Paoli P, Carbone A (October 2013). "Carcinogenic viruses and solid cancers without sufficient evidence of causal association". International Journal of Cancer. 133 (7): 1517–29. doi:10.1002/ijc.27995. PMID 23280523. S2CID 38402898.
- ↑ Anand P, Kunnumakkara AB, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (September 2008). "Cancer is a preventable disease that requires major lifestyle changes". Pharmaceutical Research. 25 (9): 2097–116. doi:10.1007/s11095-008-9661-9. PMC 2515569. PMID 18626751.
- ↑ "Human Papillomavirus (HPV) and Cancer". CDC. 2 January 2018. Retrieved 22 March 2018.
- ↑ Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K (November 2004). "Mechanisms of human papillomavirus-induced oncogenesis". Journal of Virology. 78 (21): 11451–60. doi:10.1128/JVI.78.21.11451-11460.2004. PMC 523272. PMID 15479788.
- 1 2 Sung MW, Thung SN, Acs G (2000). Hepatitis Viruses. BC Decker.
- ↑ Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, Roizman B (December 2004). "Infectious agents and cancer: criteria for a causal relation". Seminars in Cancer Biology. 14 (6): 453–71. doi:10.1016/j.semcancer.2004.06.009. PMID 15489139.
- ↑ Hatakeyama, Masanori (9 December 2005). "Helicobacter pylori CagA: a new paradigm for bacterial carcinogenesis". Cancer Science. 96 (12): 835–843. doi:10.1111/j.1349-7006.2005.00130.x. PMID 16367902.
- ↑ Samaras V, Rafailidis PI, Mourtzoukou EG, Peppas G, Falagas ME (June 2010). "Chronic bacterial and parasitic infections and cancer: a review" (PDF). Journal of Infection in Developing Countries. 4 (5): 267–81. doi:10.3855/jidc.819. PMID 20539059.
- ↑ Mustacchi, Piero (2000). Parasites. BC Decker.
- ↑ Pallis AG, Syrigos KN (December 2013). "Lung cancer in never smokers: disease characteristics and risk factors". Critical Reviews in Oncology/Hematology. 88 (3): 494–503. doi:10.1016/j.critrevonc.2013.06.011. PMID 23921082.
- ↑ Taniguchi K, Karin M (February 2014). "IL-6 and related cytokines as the critical lynchpins between inflammation and cancer". Seminars in Immunology. 26 (1): 54–74. doi:10.1016/j.smim.2014.01.001. PMID 24552665.
- ↑ Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (July 2009). "Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability". Carcinogenesis. 30 (7): 1073–81. doi:10.1093/carcin/bgp127. PMID 19468060.
- ↑ Mantovani A (June 2010). "Molecular pathways linking inflammation and cancer". Current Molecular Medicine. 10 (4): 369–73. doi:10.2174/156652410791316968. PMID 20455855.
- 1 2 3 4 5 6 7 8 9 10 11 12 Little JB (2000). "Chapter 14: Ionizing Radiation". In Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei E (eds.). Cancer medicine (6th ed.). Hamilton, Ont: B.C. Decker. ISBN 978-1-55009-113-7.
- ↑ "IARC classifies radiofrequency electromagnetic fields as possibly carcinogenic to humans" (PDF). World Health Organization.
- ↑ "IARC Monographs- Classifications". monographs.iarc.fr. Archived from the original on 10 June 2017. Retrieved 13 March 2018.
- ↑ "Cell Phones and Cancer Risk - National Cancer Institute". Cancer.gov. 8 May 2013. Retrieved 15 December 2013.
- 1 2 Cleaver JE, Mitchell DL (2000). "15. Ultraviolet Radiation Carcinogenesis". In Bast RC, Kufe DW, Pollock RE, et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 978-1-55009-113-7. Retrieved 31 January 2011.
- ↑ Yamanaka R, Hayano A, Kanayama T (January 2017). "Radiation-Induced Meningiomas: An Exhaustive Review of the Literature". World Neurosurgery. 97: 635–644.e8. doi:10.1016/j.wneu.2016.09.094. PMID 27713063.
- ↑ Brenner DJ, Hall EJ (November 2007). "Computed tomography--an increasing source of radiation exposure". The New England Journal of Medicine. 357 (22): 2277–84. doi:10.1056/NEJMra072149. PMID 18046031. S2CID 2760372.
- ↑ Dingli D, Nowak MA (September 2006). "Cancer biology: infectious tumour cells". Nature. 443 (7107): 35–6. Bibcode:2006Natur.443...35D. doi:10.1038/443035a. PMC 2711443. PMID 16957717.
- ↑ Barozzi P, Luppi M, Facchetti F, Mecucci C, Alù M, Sarid R, Rasini V, Ravazzini L, Rossi E, Festa S, Crescenzi B, Wolf DG, Schulz TF, Torelli G (May 2003). "Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors". Nature Medicine. 9 (5): 554–61. doi:10.1038/nm862. PMID 12692543. S2CID 2527251.
- 1 2 3 4 5 6 Gaeta JF (2000). "Chapter 17: Trauma and Inflammation". In Bast RC, Kufe DW, Pollock RE, et al. (eds.). Holland-Frei Cancer Medicine (5th ed.). Hamilton, Ontario: B.C. Decker. ISBN 978-1-55009-113-7. Retrieved 27 January 2011.
- 1 2 Tolar J, Neglia JP (June 2003). "Transplacental and other routes of cancer transmission between individuals". Journal of Pediatric Hematology/Oncology. 25 (6): 430–4. doi:10.1097/00043426-200306000-00002. PMID 12794519.