 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Norpace |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682408 |
| Pregnancy category |
|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 50% to 65% (concentration-dependent) |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Elimination half-life | 6.7 hours (range 4 to 10 hours) |
| Excretion | Renal (80%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.010 |
| Chemical and physical data | |
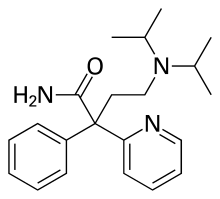
| Formula | C21H29N3O |
| Molar mass | 339.483 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 94.5 to 95 °C (202.1 to 203.0 °F) |
| |
| |
| (verify) | |
Disopyramide (INN, trade names Norpace and Rythmodan) is an antiarrhythmic medication used in the treatment of ventricular tachycardia.[2] It is a sodium channel blocker and is classified as a Class 1a anti-arrhythmic agent.[3][4] Disopyramide has a negative inotropic effect on the ventricular myocardium, significantly decreasing the contractility.[5][6] Disopyramide also has an anticholinergic effect on the heart which accounts for many adverse side effects. Disopyramide is available in both oral and intravenous forms, and has a low degree of toxicity.[6]
Mechanism of action
Disopyramide's Class 1a activity is similar to that of quinidine in that it targets sodium channels to inhibit conduction.[4][6] Disopyramide depresses the increase in sodium permeability of the cardiac myocyte during Phase 0 of the cardiac action potential, in turn decreasing the inward sodium current. This results in an increased threshold for excitation and a decreased upstroke velocity.[4] Disopyramide prolongs the PR interval by lengthening both the QRS and P wave duration.[6] This effect is particularly well suited in the treatment of ventricular tachycardia as it slows the action potential propagation through the atria to the ventricles. Disopyramide does not act as a blocking agent for beta or alpha adrenergic receptors, but does have a significant negative inotropic effect on the ventricular myocardium.[7] As a result, the use of disopyramide may reduce contractile force up to 42% at low doses and up to 100% in higher doses compared to quinidine.[6]
Levites proposed a possible secondary mode of action for disopyramide, against reentrant arrhythmias after an ischemic insult. Disopyramide decreases the inhomogeneity between infarcted and normal myocardium refractory periods; in addition to lengthening the refractory period.[5] This decreases the chance of re-entry depolarization, because signals are more likely to encounter tissue in a refractory state which cannot be excited.[2] This provides a possible treatment for atrial and ventricular fibrillation, as it restores pacemaker control of the tissue to the SA and AV nodes.[8]
Obstructive hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disease, occurring in 1:500 individuals in the general population. It is estimated that there are 600,000 individuals in the United States with hypertrophic cardiomyopathy. The most common variant of HCM presents with left ventricular (LV) intracavitary obstruction due to systolic anterior motion of the mitral valve, and mitral-septal contact, diagnosed readily with echocardiography. Pharmacologic treatment with negative inotropic drugs is first-line therapy. Beta-blockers are used first, and while they improve symptoms of shortness of breath, chest pain and exercise intolerance, they do not reduce resting LV intraventricular pressure gradients and often are inadequate to control symptoms. Many investigators and clinicians believe that disopyramide controlled release is the most potent agent available for reducing resting pressure gradients and improving symptoms.[9][10][11][12] Disopyramide has been actively used for more than 30 years.[13] Disopyramide administration for obstructive HCM has a IB recommendation in the 2020 American Heart Association/American College of Cardiology Foundation guidelines for treatment of obstructive HCM.[14] A IB treatment recommendation indicates that a treatment is recommended, and may be useful, and beneficial.
Negative inotropes improve left ventricular (LV) obstruction by decreasing LV ejection acceleration and hydrodynamic forces on the mitral valve. Disopyramide's particular efficacy is due to its potent negative inotropic effects; in head-to-head comparison, it is more effective for gradient reduction than either beta-blocker or verapamil.[15] Disopyramide is most often administered with beta-blockade. When used in patients resistant to beta-blockade, disopyramide is effective in 60% of cases, reducing symptoms and gradient to the extent that invasive procedures such as surgical septal myectomy are not required.[12]
Disopyramide, despite its efficacy, has one main side effect that has limited its use in the US, though it has seen wider application in Canada, UK and Japan. Vagal blockade predictably causes dry mouth, and in men with prostatism, may cause urinary retention. Teichman et al. showed that pyridostigmine used in combination with disopyramide substantially alleviates vagolytic side effects without compromising antiarrhythmic efficacy.[16] This combination has also been shown to be effective and safe in obstructive HCM in a large cohort of patients.[12] Some clinicians prescribe pyridostigmine sustained release (marketed in the US as Mestinon Timespan) to every patient begun on disopyramide.[17] This combination increases acceptance of higher disopyramide dosing, important since there is a dose-response correlation in obstructive HCM, higher doses yielding lower gradients.
Another concern about disopyramide has been the hypothetical potential for inducing sudden death from its type 1 anti-arrhythmic effects. However, a multicenter registry and two recent cohort registries have largely reduced this concern, by showing sudden death rates lower than that observed from the disease itself.[9][10][12]
These concerns about the drug must be viewed from the clinical perspective that disopyramide is generally the last agent that is tried for patients before they are referred for invasive septal reduction with surgical septal myectomy (an open-heart operation) or alcohol septal ablation (a controlled heart attack). Both of these invasive procedures have risk of morbidity and mortality.
For selected patients, a trial of oral disopyramide is a reasonable approach before proceeding to invasive septal reduction. Patients who respond to disopyramide are continued on the drug. Those who continue to have disabling symptoms or who experience side effects are promptly referred for septal reduction. Using such a stepped strategy, investigators have reported that survival does not differ from that observed in the age-matched normal United States population.[12]
Side effects
Disopyramide has the following side effects:[18]
- Mild side effects
- Dry mouth
- Polyuria (frequent urination)
- Constipation
- Blurred vision
- Rash
- Bloating
- Dizziness
- Fatigue
- Serious side effects
Adverse effects
Cardiac adverse effects
- Acute decompensated heart failure]: Disopyramide should not be given to patients with impaired left ventricular (LV) systolic function and low ejection fraction. Heart failure is not seen when disopyramide is used in patients with normal or supernormal LV systolic function.
- Severe hypotension – Disopyramide should not be given to patients with impaired LV systolic function and low ejection fraction. Hypotension is not seen in patients with normal or supernormal LV systolic function.
Extracardiac adverse effects
Disopyramide has atropine-like anticholinergic effects.[19]
- Urinary retention – Disopyramide should not be given to patients with symptomatic prostatism.
- Glaucoma
- Agranulocytosis
Additionally, disopyramide may enhance the hypoglycemic effect of gliclazide, insulin, and metformin.
See also
References
- ↑ "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control]. Diário Oficial da União [Official Diary of the Union] (in Brazilian Portuguese). Brazilian Health Regulatory Agency (Anvisa) (published 2023-04-04). 2023-03-31. Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- 1 2 Hall, John Edward; Guyton, Arthur C. (2006). Pocket Companion to Guyton & Hall Textbook of Medical Physiology. Elsevier. ISBN 9788480862325. OCLC 1027935564.
- ↑ Rizos I, Brachmann J, Lengfelder W, Schmitt C, von Olshausen K, Kübler W, Senges J (February 1987). "Effects of intravenous disopyramide and quinidine on normal myocardium and on the characteristics of arrhythmias: intraindividual comparison in patients with sustained ventricular tachycardia". European Heart Journal. 8 (2): 154–163. doi:10.1093/oxfordjournals.eurheartj.a062243. PMID 3569310.
- 1 2 3 Kim SY, Benowitz NL (1990). "Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide". Drug Safety. 5 (6): 393–420. doi:10.2165/00002018-199005060-00002. PMID 2285495. S2CID 71415838.
- 1 2 Levites R, Anderson GJ (September 1979). "Electrophysiological effects of disopyramide phosphate during experimental myocardial ischemia". American Heart Journal. 98 (3): 339–344. doi:10.1016/0002-8703(79)90046-2. PMID 474380.
- 1 2 3 4 5 Mathur PP (December 1972). "Cardiovascular effects of a newer antiarrhythmic agent, disopyramide phosphate". American Heart Journal. 84 (6): 764–770. doi:10.1016/0002-8703(72)90069-5. PMID 4150336.
- ↑ Hulting J, Rosenhamer G: Hemodynamic and electrocardiographic effects of disopyramide in patients with ventricular arrhythmia. Acta Med Scand 199:41-51, 1976.
- ↑ Katzung BG, Masters SB, Trevor AJ (2009). Basic and Clinical Pharmacology (11th ed.). New York: McGraw Hill.
- 1 2 Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph-Edwards A, et al. (November 2011). "Long-term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment". Journal of the American College of Cardiology. 58 (22): 2313–2321. doi:10.1016/j.jacc.2011.08.040. PMID 22093509.
- 1 2 Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, et al. (April 2005). "Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 45 (8): 1251–1258. doi:10.1016/j.jacc.2005.01.012. PMID 15837258.
- ↑ Elliott PM, Gimeno JR, Thaman R, Shah J, Ward D, Dickie S, et al. (June 2006). "Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy". Heart. 92 (6): 785–791. doi:10.1136/hrt.2005.068577. PMC 1860645. PMID 16216855.
- 1 2 3 4 5 Sherrid MV, Shetty A, Winson G, Kim B, Musat D, Alviar CL, et al. (July 2013). "Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with β-blockade or verapamil". Circulation: Heart Failure. 6 (4): 694–702. doi:10.1161/CIRCHEARTFAILURE.112.000122. PMID 23704138.
- ↑ Pollick C (October 1982). "Muscular subaortic stenosis: hemodynamic and clinical improvement after disopyramide". The New England Journal of Medicine. 307 (16): 997–999. doi:10.1056/nejm198210143071607. PMID 7202121.
- ↑ Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. (December 2020). "2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines". Journal of the American College of Cardiology. 76 (25): e159–e240. doi:10.1016/j.jacc.2020.08.045. PMID 33229116.
- ↑ Kajimoto K, Imai T, Minami Y, Kasanuki H. Comparison of acute reduction in left ventricular outflow tract pressure gradient in obstructive hypertrophic cardiomyopathy by disopyramide versus pilsicainide versus cibenzoline. Am J Cardiol. 2010;106:1307-1312
- ↑ Teichman SL, Ferrick A, Kim SG, Matos JA, Waspe LE, Fisher JD (September 1987). "Disopyramide-pyridostigmine interaction: selective reversal of anticholinergic symptoms with preservation of antiarrhythmic effect". Journal of the American College of Cardiology. 10 (3): 633–641. doi:10.1016/s0735-1097(87)80207-3. PMID 3624669.
- ↑ Sherrid MV, Arabadjian M. A primer of disopyramide treatment of obstructive hypertrophic cardiomyopathy. Prog Cardiovasc Dis. 2012;54:483-492
- ↑ "Disopyramide - Drug Information | MedlinePlus". medlineplus.gov. Retrieved 2023-12-12.
- ↑ Ritter, James M.; Flower, Rod; Henderson, Graeme; Loke, Yoon Kong; MacEwan, David; Robinson, Emma; Fullerton, James (2023-05-24). Rang and Dale's Pharmacology. Elsevier. ISBN 9780323873956. OCLC 1362865747.