 | |
| Names | |
|---|---|
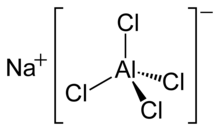
| IUPAC name
Sodium chloroaluminate | |
| Other names
Natrium chloroaluminate, Sodium tetrachloroaluminate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.136 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| NaAlCl4 | |
| Molar mass | 191.78331 g/mol |
| Melting point | 157 °C |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H314 | |
| P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P405, P501 | |
| Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium tetrachloroaluminate, also known as natrium chloroaluminate, is a chemical compound with the formula NaAlCl4. It was discovered in the 20th century. It is the sodium salt of the tetrachloroaluminate anion.
Sodium tetrachloroaluminate can be prepared from sodium chloride and aluminium trichloride.
Uses
Molten sodium tetrachloroaluminate is used as an electrolyte in sodium-nickel chloride batteries.
See also
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.