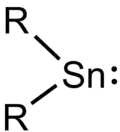
Stannylenes (R2Sn:) are a class of organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes have a singlet ground state since valence orbitals of tin (Sn) have less tendency to form hybrid orbitals and thus the electrons in 5s orbital are still paired up.[1] Free stannylenes are stabilized by steric protection. Adducts with Lewis bases are also known.[2]
History
The first persistent stannylene, [(Me3Si)2CH]2Sn, was reported by Michael F. Lappert in 1973.[3] Lappert used the same synthetic approach to synthesize the first diamidostannylene [(Me3Si)2N]2Sn in 1974.[4]
The short-lived, transient stannylene Me2Sn has been generated by thermolysis of cyclo-(Me2Sn)6.[5]
Synthesis and characterization
Persistent stannylene
Most alkyl stannylenes have been synthesized by alkylation of tin(II) dihalides with organolithium reagents. For example, the first stannylene, [(Me3Si)2CH]2Sn, was synthesized using (Me3Si)2CHLi and SnCl2.[3]
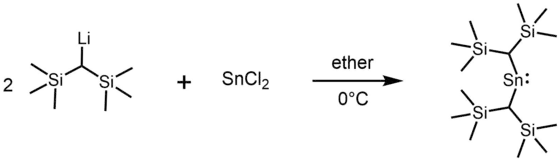
In some cases, stannylenes have been prepared by reduction of a tin(IV) compound by KC8[6]
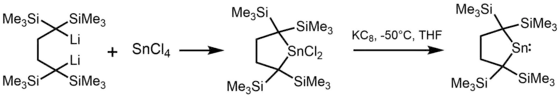
Amidostannylene can also be synthesized by using a tin(II) dihalide and the lithium amide.[4]
Short-lived stannylene
The isolation of a transient alkyl stannylene is more difficult. The first isolation of dimethylstannylene was believed to be done by thermolysing cyclostannane (Me2Sn)6, which was the product of the condensation of Me2Sn(NEt2)2 and Me2SnH2. The evidence came from the vibrational frequencies of dimethylstannylene identified by infrared spectroscopy, which is consistent with the calculated value.[5] The existence of this elusive SnMe2 was further confirmed by the discovery of visible light absorption matching the calculated electronic transition of SnMe2 in gas phase.[7]
Another method to prepare short-lived stannylene is laser flash photolysis using tetraalkyltin(IV) compound (e.g. SnMe4) as a precursor. The generation of stannylene can be monitored by transient UV-VIS spectroscopy.[8]
Structure and bonding
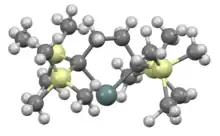
Stannylenes can be viewed as sp2-hybridized with vacant 5p orbital and a lone pair. This gives rise to their red color from n to p transition.[3][9]
With specific type of ligands, the electron deficiency of monomeric stannylene is reduced by the agostic interaction from B-H bond. This concept was proved by Mark Kenyon and coworkers in 2006 when they synthesized the cyclic dialkylstannylene [{n-Pr2P(BH3)}(Me3Si)CCH2]2Sn. The crystal structure of the synthesized compound showed the arrangement of one B-H bond toward the Sn atom with the B—H--Sn bond distance of 2.03 Å. The mitigation of Sn electron deficiency was proved by the spectroscopic data, especially the 119Sn NMR spectra which showed the drastically low chemical shift (587 and 787 ppm comparing to 2323 ppm in analogous dialkylstannylene) indicating more electron density around Sn in this case.[10]
Reactivity
Oligomerization
Small, unstable stannylenes (e.g. dimethylstannylene) undergo self-oligomerization yielding cyclic oligostannanes, which can be used as stannylene sources.[5]
More bulky stannylenes (e.g. Lappert's stannylene), on the other hand, tend to form a dimer. The nature of the Sn-Sn bond in stannylene dimer is rather different from C-C bond in carbene dimer (i.e. alkene). As alkene develops a typical double bond character and the molecule has a planar geometry, stannylene dimer has a trans-bent geometry. The double bond in stannylene dimer can be considered as two donor-acceptor interactions. The electron localization function (ELF) analysis of stannylene dimer shows a disynaptic basin (electrons in bonding orbitals) on both Sn atom, indicating that the interaction between two Sn atom is two unusual bent dative bonds.[11] Apart from that, the stability of stannylene dimer is also affected by the steric repulsion and dispersion attraction between bulky substituents.[12]

Insertion reaction
Alkylstannylenes can react with various reagents (e.g. alkyl halides, enones, dienes) in an oxidative addition (or insertion) fashion. The reaction between stannylene and 9,10-phenanthrolinedione produces an EPR signal that was identified to be 9,10-phenanthrenedione radical anion, indicating that this reaction proceeds via radical mechanism.[13]

Cycloaddition
Although stannylenes are more stable than its lighter carbene analogs, they readily undergo [2+4] cycloaddition reaction with Z-alkenes. The addition of (CH(SiMe3)2)2Sn, to 2,7-diphenylocta-2,3,5,6-tetraene proceeds in a cheletropic fashion, as allowed by Woodward-Hoffmann rules.[14]

Metal center for oxidative addition and reductive elimination
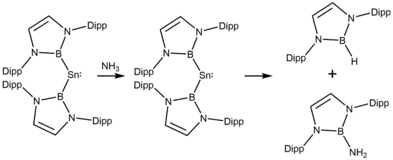
In terms of the SnII/SnIV couple, certain stannylenes resemble transition metals. The singlet-triplet energy separation is considered to have a strong effect on oxidative addition reactivity,[15] by utilizing a very strong σ-donor boryl (-BX2) ligand. The results demonstrated that not only molecular hydrogen but also E-H bond (E = B, Si, O, N) can be oxidative added on Sn. In ammonia and water cases, the oxidative added product could also undergo reductive elimination, yielding O- or N-B bond formation.[16]
See also
References
- ↑ Sasamori, T.; Tokitoh, N. (2005). Encyclopedia of Inorganic Chemistry II. Chichester, U.K.: John Wiley & Sons. pp. 1698–1740.
- ↑ Mizuhata, Yoshiyuki; Sasamori, Takahiro; Tokitoh, Norihiro (2009). "Stable Heavier Carbene Analogues". Chemical Reviews. 109 (8): 3479–3511. doi:10.1021/cr900093s. PMID 19630390.
- 1 2 3 Davidson, Peter J.; Lappert, Michael F. (1973). "Stabilisation of Metals in a Low Co-ordinative Environment using the Bis(trimethylsilyl)methyl Ligand; Coloured SnII and PbII Alkyls, M[(Me3Si)2CH]2". Journal of the Chemical Society, Chemical Communications (9): 317. doi:10.1039/C3973000317A.
- 1 2 Harris, David H.; Lappert, Michael F. (1974). "Monomeric, volatile bivalent amides of group IVB elements, M(NR12)2 and M(NR1R2)2(M=Ge, Sn, or Pb; R1=Me3Si, R2=Me3C)". Journal of the Chemical Society, Chemical Communications (21): 895–896. doi:10.1039/C39740000895.
- 1 2 3 Bleckmann, Paul; Maly, Hartwig; Minkwitz, Rolf; Watta, Barbel; Neumann, Wilhelm P. (1982). "Matrix Isolation and IR Spectroscopy of Stannylenes (CH3)2Sn and (CD3)2Sn". Tetrahedron Letters. 23 (45): 4655–4658. doi:10.1016/s0040-4039(00)85679-8.
- ↑ Kira, Mitsuo; Ishida, Shintaro; Iwamoto, Takeaki; Yauchibara, Rika; Sakurai, Hideki (2001). "New synthesis of a stable dialkylstannylene and its reversible complexation with tetrahydrofuran". Journal of Organometallic Chemistry. 636 (1–2): 144–147. doi:10.1016/s0022-328x(01)00998-6.
- ↑ Walsh, Robin (2002). "First Gas-Phase Detection of Dimethylstannylene and Time-Resolved Study of Some of Its Reactions". Journal of the American Chemical Society. 124 (25): 7555–7562. doi:10.1021/ja012691k. PMID 12071766.
- ↑ Becerra, Rosa; Gaspar, Peter P.; Harrington, Cameron; Leigh, William J.; Vargas-Baca, Ignacio; Walsh, Robin; Zhou, Dong (2005). "Direct Detection of Dimethylstannylene and Tetramethyldistannene in Solution and the Gas Phase by Laser Flash Photolysis of 1,1-Dimethylstannacyclopent-3-enes". Journal of the American Chemical Society. 127 (49): 17469–17478. doi:10.1021/ja052675d. PMID 16332099.
- ↑ Davies, Alwyn G. (2004). Organotin Chemistry. Weinheim: Wiley-VCH. p. 364. ISBN 3-527-31023-1.
- ↑ Izod, Keith (2006). "Stabilization of a Dialkylstannylene by Unusual B-HâââSn ç-Agostic-Type Interactions. A Structural, Spectroscopic, and DFT Study". Organometallics. 25: 1135–1143. doi:10.1021/om0600036.
- ↑ Popelier, Paul L. A.; Malcolm, Nathaniel O.J.; Gillispie, Ronald J. (2002). "A topological study of homonuclear multiple bonds between the elements of group 14". Journal of the Chemical Society, Dalton Transactions (17): 3333–3341. doi:10.1039/B110610B.
- ↑ Růžička, Aleš; Hobza, Pavel (2016). "New Insight into the Nature of Bonding in the Dimers of Lappert's Stannylene and Its Ge Analogs: A Quantum Mechanical Study". Journal of Chemical Theory and Computation. 23 (4): 1696–1704. doi:10.1021/acs.jctc.6b00065. PMID 26953594.
- 1 2 Eaborn, Colin; Smith, J. David (2002). "Reactions of a Highly Crowded Cyclic Stannylene with Iodoalkanes, Enones, and Dienes. Inhibition of Nucleophilic Substitution at Tin(IV) Centers". Organometallics. 21 (12): 2430–2437. doi:10.1021/om020106y.
- ↑ Neumann, Wilhelm P. (1984). "[2+4] Cheletropic cycloaddition of stannylenes R2Sn". Tetrahedron Letters. 25 (6): 625–628. doi:10.1016/S0040-4039(00)99955-6.
- ↑ Wang, Yong; Ma, Jing (2009). "Silylenes and germylenes: The activation of H–H bond in hydrogen molecule". Journal of Organometallic Chemistry. 694 (16): 2567–2575. doi:10.1016/j.jorganchem.2009.03.051.
- 1 2 Protchenko, Andrey; Aldridge, Simon (2016). "Enabling and Probing Oxidative Addition and Reductive Elimination at a Group 14 Metal Center: Cleavage and Functionalization of E−H Bonds by a Bis(boryl)stannylene". Journal of the American Chemical Society. 138 (13): 4555–4564. doi:10.1021/jacs.6b00710. PMID 26981766.