 | |
| Names | |
|---|---|
| Other names
SAN | |
| Identifiers | |
| ChemSpider |
|
| ECHA InfoCard | 100.127.519 |
| EC Number |
|
CompTox Dashboard (EPA) |
|
| Properties | |
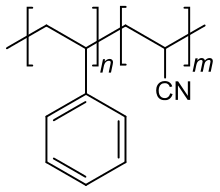
| (C8H8)n-(C3H3N)m | |
| Molar mass | variable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Styrene acrylonitrile resin (SAN) is a copolymer plastic consisting of styrene and acrylonitrile. It is widely used in place of polystyrene owing to its greater thermal resistance. The chains of between 70 and 80% by weight styrene and 20 to 30% acrylonitrile.[1] Larger acrylonitrile content improves mechanical properties and chemical resistance, but also adds a yellow tint to the normally transparent plastic.[2]
Properties
SAN is similar in use to polystyrene. Like polystyrene itself, it is optically transparent and brittle in mechanical behavior. The copolymer has a glass transition temperature greater than 100 °C owing to the acrylonitrile units in the chain, thus making the material resistant to boiling water. It is structurally related to ABS plastic, where polybutadiene is copolymerised with SAN to give a much tougher material. The rubber chains form separate phases which are 10-20 micrometers in diameter. When the product is stressed, crazing from the particles helps to increase the strength of the polymer. The method of rubber toughening has been used to strengthen other polymers such as PMMA and nylon.
Uses
Uses include food containers, water bottles, kitchenware, e.g., blenders and mixers, healthcare materials, cosmetic jars, computer products, packaging material, household equipment e.g., shower trays, battery cases and plastic optical fibers.
Health risks
The acrylonitrile from SAN containers has been found to migrate to content in variable amounts.[3] Acrylonitrile is classified as a Class 2B carcinogen (possibly carcinogenic) by the International Agency for Research on Cancer (IARC).[4] Acrylonitrile has been shown to increase rates of cancer appearance in high dosage tests in male and female rats and mice.[5]
References
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Vol 29. Polystyrene and Styrene Copolymers. Wiley Online Library. p. 487
- ↑ Charles A. Harper, ed., Modern Plastics Handbook, ISBN 0-07-026714-6, 2000.
- ↑ http://www.agriculturejournals.cz/publicFiles/00366.pdf
- ↑ "Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide". IARC Monographs, Volume 71 (1999)
- ↑ "Acrylonitrile: Carcinogenic Potency Database". berkeley.edu.

