 | |
| Clinical data | |
|---|---|
| Trade names | Vantas, Supprelin LA, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601146 |
| Routes of administration | Subcutaneous implant |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 92% |
| Protein binding | 70% |
| Metabolism | Hepatic |
| Elimination half-life | 4.0 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.163.860 |
| Chemical and physical data | |
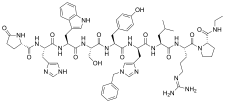
| Formula | C66H86N18O12 |
| Molar mass | 1323.528 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Histrelin acetate, sold under the brand names Vantas and Supprelin LA among others, is a nonapeptide analogue of gonadotropin-releasing hormone (GnRH) with added potency.[1] When present in the bloodstream, it acts on particular cells of the pituitary gland called gonadotropes. Histrelin stimulates these cells to release luteinizing hormone and follicle-stimulating hormone. Thus it is considered a gonadotropin-releasing hormone agonist or GnRH agonist.
Medical uses
Histrelin is used to treat hormone-sensitive cancers of the prostate in men and uterine fibroids in women. In addition, histrelin has been proven to be highly effective in treating central precocious puberty in children.[2][3]
It is available as a daily intramuscular injection.
Histrelin is also available in a 12-month subcutaneous implant (Vantas) for the palliative treatment of advanced prostate cancer, since 2005 in the US, and since Jan 2010 in the UK.
A 12-month subcutaneous implant (Supprelin LA) for central precocious puberty (CPP) was approved on May 3, 2007 by the U.S. Food and Drug Administration.
Histrelin can be part of the primary care protocol in transgender children/youth, which is an off-label use in the USA[4] and the UK,[5] and is used in suppressing cis-sex puberty, until the patient is ready to begin cross-sex hormonal therapy. It is also sometimes prescribed to transgender adults who benefit from having their sex hormone production halted. In this application, patients often keep the implant for two years before replacing, with regular blood tests to monitor the hormone levels. Implants left too long are more difficult to replace.[6]
Since 2020, Vantas is not available anymore. Endo, its manufacturer said batches of the medication were not coming out right. They added that although Supprelin and Vantas were manufactured in the same facility, they were not identical product (though both contain 50 mg of histrelin acetate.) As of 2023, Vantas is still not available and the only available implant is Supprelin LA. [7]
Vantas was priced around $4400 in 2004 while Supprelin LA was priced at $37,000 in 2007.[8]
Side effects
Common side effects include headache, hot flashes, constipation, reduced libido, gynecomastia, insomnia, renal impairment, weight loss, testicular atrophy, and erectile dysfunction.[9]
Pharmacology
In a process known as downregulation, daily stimulation of pituitary gonadotropes causes them to become desensitized to the effects of histrelin. As a consequence, levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) fall after a short period of time. From that point forward, as long as histrelin is administered, the levels of LH and FSH in the blood remain low.[10][11]
This prolonged lowering of LH and FSH levels is the rationale for therapy using GnRH agonists. Since LH and FSH stimulate the gonads to produce estrogens and androgens in females and males respectively, histrelin can effectively be used to decrease the sex steroids in the blood of patients.
See also
References
- ↑ Davis M (25 February 2005). Chandramouli J (ed.). "Histrelin acetate (Vantas)". New Drug Bulletins. University of Utah Hospitals & Clinics. Archived from the original on 10 May 2006.
- ↑ "Histrelin consumer information". Drugs.com.
- ↑ Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. (May 2007). "Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial". The Journal of Clinical Endocrinology and Metabolism. 92 (5): 1697–1704. doi:10.1210/jc.2006-2479. PMID 17327379.
- ↑ "Primary Care Protocol for Transgender Patient Care: Hormone Administration". Center of Excellence for Transgender Health. University of California, San Francisco, Department of Family and Community Medicine. April 2011. Archived from the original on 2012-01-17. Retrieved 2015-09-29.
- ↑ Cohen D, Barnes H (September 2019). "Gender dysphoria in children: puberty blockers study draws further criticism". BMJ. 366: l5647. doi:10.1136/bmj.l5647. PMID 31540909. S2CID 202711942.
- ↑ Marinkovic M, Carswell J, Roberts SA (2019). "Emerging Developments in Pubertal Suppression for Gender Incongruent/Gender Dysphoric Youth". In Finlayson C (ed.). Pubertal Suppression in Transgender Youth. pp. 95–100. doi:10.1016/B978-0-323-56963-7.00012-0. ISBN 978-0-323-56963-7. S2CID 186736821.
- ↑ Lupkin S (6 November 2021). "Drugmaker drops cheaper version of drug, leaving patients stuck with pricier one". National Public Radio (NPR).
- ↑ Lupkin S (24 February 2020). "Hormone Blocker Sticker Shock: Kids Drug Costs 8 Times More Than One For Adults". National Public Radio (NPR).
- ↑ Drugs.com: Histrelin Monograph
- ↑ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 372–3. ISBN 3-8047-1763-2.
- ↑ Wuttke W, Jarry H, Feleder C, Moguilevsky J, Leonhardt S, Seong JY, Kim K (1996). "The neurochemistry of the GnRH pulse generator". Acta Neurobiologiae Experimentalis. 56 (3): 707–713. PMID 8917899.
External links
- "Central Precocious Puberty". Endo Pharmaceuticals. June 2009. Archived from the original on 2011-07-16.