 | |
| Names | |
|---|---|
| IUPAC name
Technetium(IV) chloride | |
| Other names
Technetium tetrachloride, Technetium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| TcCl4 | |
| Molar mass | 239.718 g/mol |
| Appearance | Red solid |
| Boiling point | 300 °C (572 °F; 573 K)[1] |
| Structure | |
| Orthorhombic, oP40 | |
| Pbca, No. 61 | |
a = 0.603 nm, b = 1.165 nm, c = 1.406 nm α = 90°, β = 90°, γ = 90° | |
| Related compounds | |
Other anions |
Technetium(VI) fluoride |
Other cations |
Manganese(II) chloride Rhenium(V) chloride Ruthenium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Technetium(IV) chloride is the inorganic compound with the formula TcCl4. It was discovered in 1957 as the first binary halide of technetium. It is the highest oxidation binary chloride of technetium that has been isolated as a solid. It is volatile at elevated temperatures and its volatility has been used for separating technetium from other metal chlorides.[2] Colloidal solutions of technetium(IV) chloride are oxidized to form Tc(VII) ions when exposed to gamma rays.[3]
Technetium tetrachloride can be synthesized from the reaction of Cl2 with technetium metal at elevated temperatures between 300 and 500 °C:[4]
- Tc + 2 Cl2 → TcCl4
Technetium tetrachloride has also been prepared from the reaction of technetium(VII) oxide with carbon tetrachloride in a sealed vessel at elevated temperature:[5]
- Tc2O7 + 7 CCl4 → 2 TcCl4 + 7 COCl2 + 3 Cl2
At 450 °C under vacuum, TcCl4 decomposes to TcCl3 and TcCl2.[6]
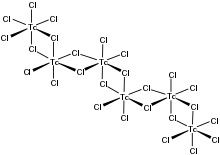
As verified by X-ray crystallography, the compound is an inorganic polymer consisting of interconnected TcCl6 octahedra.
References
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Schwochau, Klaus (2000). Technetium. Wiley-VCH. p. 67. ISBN 978-3-527-29496-1.
- ↑ Fattahi, M.; Vichot, L.; Poineau, F.; Houée-Levin, C.; Grambow, B. (2005). "Speciation of technetium(IV) chloride under gamma irradiation". Radiochimica Acta. 93 (7): 409–413. doi:10.1524/ract.2005.93.7.409. S2CID 96640348.
- ↑ Johnstone, Erik V.; Poineau, Frederic; Forster, Paul M.; Ma, Longzou; Hartmann, Thomas; Cornelius, Andrew; Antonio, Daniel; Sattelberger, Alfred P.; Czerwinski, Kenneth R. (2012-07-09). "Technetium Tetrachloride Revisited: A Precursor to Lower-Valent Binary Technetium Chlorides". Inorganic Chemistry. 51 (15): 8462–8467. doi:10.1021/ic301011c. OSTI 1307429. PMID 22775538.
- ↑ Housecroft, Catherine E.; Sharpe, A. G. (2005). Inorganic chemistry. Upper Saddle River, N.J.: Pearson Prentice Hall. p. 669. ISBN 0-13-039913-2. OCLC 56834315.
- ↑ Poineau, Frederic; Johnstone, Erik V.; Czerwinski, Kenneth R.; Sattelberger, Alfred P. (2014). "Recent Advances in Technetium Halide Chemistry". Accounts of Chemical Research. 47 (2): 624–632. doi:10.1021/ar400225b. PMID 24393028.