| |||
_chloride_hexahydrate.jpg.webp) | |||
| Names | |||
|---|---|---|---|
| Other names
terbium trichloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ECHA InfoCard | 100.030.108 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| TbCl3 | |||
| Molar mass | 265.2834 g/mol | ||
| Appearance | white powder | ||
| Density | 4.35 g/cm3, solid | ||
| Melting point | 558 °C (1,036 °F; 831 K) | ||
| Boiling point | 180 to 200 °C (356 to 392 °F; 453 to 473 K) (in HCl gas atmosphere) | ||
| soluble | |||
| Structure | |||
| hexagonal (UCl3 type), hP8 | |||
| P63/m, No. 176 | |||
| Tricapped trigonal prismatic (nine-coordinate) | |||
| Hazards | |||
| GHS labelling:[1] | |||
 | |||
| Warning | |||
| H315, H319 | |||
| P302+P352, P305+P351+P338 | |||
| Related compounds | |||
Other anions |
Terbium(III) oxide | ||
Other cations |
Gadolinium(III) chloride Dysprosium(III) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
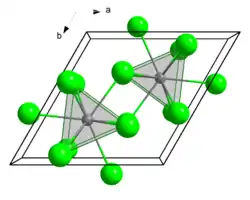
Terbium(III) chloride (TbCl3) is a chemical compound. In the solid state TbCl3 has the YCl3 layer structure.[2] Terbium(III) chloride frequently forms a hexahydrate.
Preparation
The hexahydrate of terbium(III) chloride can be obtained by the reaction of terbium(III) oxide and hydrochloric acid:[3]
- Tb2O3 + 6 HCl → 2 TbCl3 + 3 H2O
It can also be obtained by direct reaction of the elements:[4]
- 2 Tb + 3 Cl2 → 2 TbCl3
Properties
Terbium(III) chloride is a white, hygroscopic powder.[5] It crystallizes in an orthorhombic plutonium(III) bromide crystal structure with space group Cmcm (No. 63).[6][7] It can form a complex Tb(gly)3Cl3·3H2O with glycine.[8]
Applications
Terbium(III) chloride is used in the semiconductor industry.[9] The hexahydrate plays an important role as an activator of green phosphors in color TV tubes and is also used in specialty lasers and as a dopant in solid-state devices.[10]
Hazards
Terbium(III) chloride causes hyperemia of the iris.[11] Conditions/substances to avoid are: heat, acids and acid fumes.
References
- ↑ GHS: Sigma-Alderich 204560
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ XU Lijuan (许丽娟), LI Yanqiu (李艳秋), LI Xia (李 夏) (2009). "Synthesis, crystal structure and characterization of one-dimension complex constructed by terbium(III) and 2-iodobenzoate". Journal of Rare Earths. 27 (3): 372–375. doi:10.1016/S1002-0721(08)60253-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Webelements: Terbium
- ↑ Lide, David R.; CRC Press, eds. (2006). CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data (87. ed., 2006-2007 ed.). Boca Raton, Fla.: CRC, Taylor & Francis. ISBN 978-0-8493-0487-3.
- ↑ Paetzold, Peter (2009-09-10). Chemie: Eine Einführung (in German). Walter de Gruyter. ISBN 978-3-11-021135-1.
- ↑ Trotter, J. (ed.). Metals. Structure reports A. Dordrecht: Reidel. ISBN 978-90-277-2385-7.
- ↑ 郑平, 陈文生, 张洪权,等. 量热法测定氯化铽甘氨酸配合物及其配离子的标准生成焓[J]. 湖北大学学报(自科版), 2011, 33(3):270-274.
- ↑ Americanelements: Terbium Chloride
- ↑ METALL RARE EARTH LIMITED: Terbium chloride
- ↑ George C. Y. Chiou (1999). Ophthalmic toxicology (2nd ed.). CRC Press. ISBN 1-56032-722-7.
_2.png.webp)