 | |
| Names | |
|---|---|
| Other names
Thallium monobromide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.029.239 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| TlBr | |
| Molar mass | 284.287 g/mol[1] |
| Appearance | yellow crystalline solid[1] |
| Density | 7.5 g/cm3[1] |
| Melting point | 460 °C (860 °F; 733 K)[1] |
| Boiling point | 819 °C (1,506 °F; 1,092 K)[1] |
| 0.59 g/L (25 °C)[1] | |
Solubility product (Ksp) |
3.71×10−6[2] |
| −63.9·10−6 cm3/mol[3] | |
Refractive index (nD) |
2.418 (0.59 µm) 2.350 (0.75 µm) 2.289 (1 µm) 1.984 (5 µm) 2.322 (20 µm)[4] |
| Structure | |
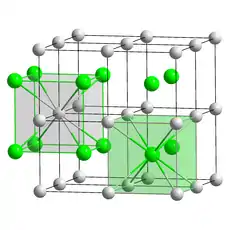
| CsCl, cP2 | |
| Pm3m, No. 221[5] | |
Formula units (Z) |
1 |
| Cubic (Tl+) Cubic (Br−) | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P321, P330, P391, P403+P233, P405, P501 | |
| Related compounds | |
Other anions |
Thallium(I) fluoride, Thallium(I) chloride, Thallium(I) iodide |
Other cations |
Indium(I) bromide, Lead(II) bromide Bismuth bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thallium(I) bromide is a chemical compound of thallium and bromine with a chemical formula TlBr. This salt is used in room-temperature detectors of X-rays, gamma-rays and blue light, as well as in near-infrared optics.
It is a semiconductor with a band gap of 2.68 eV.[6]
The crystalline structure is of cubic CsCl type at room temperature, but it lowers to the orthorhombic thallium iodide type upon cooling, the transition temperature being likely affected by the impurities.[7] Nanometer-thin TlBr films grown on LiF, NaCl or KBr substrates exhibit a rocksalt structure.[5]
Thallium is extremely toxic and a cumulative poison which can be absorbed through the skin. Acute and chronic effects of ingesting thallium compounds include fatigue, limb pain, peripheral neuritis, joint pain, loss of hair, diarrhea, vomiting, vision loss, and damage to central nervous system, liver and kidneys.[8]
References
- 1 2 3 4 5 6 Haynes, p. 4.94
- ↑ John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1138561632.
- ↑ Haynes, p. 4.135
- ↑ Haynes, p. 10.242
- 1 2 Schulz, L. G. (1951). "Polymorphism of cesium and thallium halides". Acta Crystallographica. 4 (6): 487–489. doi:10.1107/S0365110X51001641.
- ↑ Temperature Dependence of Spectroscopic Performance of Thallium Bromide X- and Gamma-Ray Detectors
- ↑ Blackman, M; Khan, I H (1961). "The Polymorphism of Thallium and Other Halides at Low Temperatures". Proceedings of the Physical Society. 77 (2): 471. Bibcode:1961PPS....77..471B. doi:10.1088/0370-1328/77/2/331.
- ↑ Thallium Bromide Material safety data sheet. espimetals.com
Cited sources
- Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. ISBN 1-4398-5511-0.