 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a682231 |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 98–99% |
| Elimination half-life | 3–8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.129 |
| Chemical and physical data | |
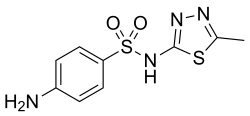
| Formula | C9H10N4O2S2 |
| Molar mass | 270.33 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 °C (406 °F) |
| |
| |
| (verify) | |
Sulfamethizole is a sulfonamide antibiotic.[1]
References
- ↑ Ayankojo AG, Tretjakov A, Reut J, Boroznjak R, Öpik A, Rappich J, et al. (January 2016). "Molecularly Imprinted Polymer Integrated with a Surface Acoustic Wave Technique for Detection of Sulfamethizole". Analytical Chemistry. 88 (2): 1476–1484. doi:10.1021/acs.analchem.5b04735. PMID 26704414.
Further reading
- Ratanajamit C, Skriver MV, Nørgaard M, Jepsen P, Schønheyder HC, Sørensen HT (November 2003). "Adverse pregnancy outcome in users of sulfamethizole during pregnancy: a population-based observational study". The Journal of Antimicrobial Chemotherapy. 52 (5): 837–841. doi:10.1093/jac/dkg438. PMID 14519675.
- Kerrn MB, Frimodt-Møller N, Espersen F (March 2003). "Effects of sulfamethizole and amdinocillin against Escherichia coli strains (with various susceptibilities) in an ascending urinary tract infection mouse model". Antimicrobial Agents and Chemotherapy. 47 (3): 1002–1009. doi:10.1128/AAC.47.3.1002-1009.2003. PMC 149286. PMID 12604534.
- Watanabe H, Hastings JW (June 1990). "Inhibition of bioluminescence in Photobacterium phosphoreum by sulfamethizole and its stimulation by thymine". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1017 (3): 229–234. doi:10.1016/0005-2728(90)90189-B. PMID 2372557.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.