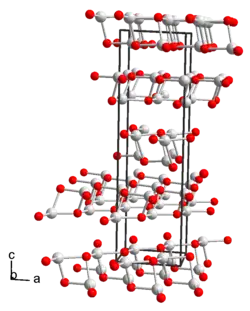
 | |
| Names | |
|---|---|
| IUPAC name
thallium(I) hydroxide | |
| Other names
thallous hydroxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.540 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| TlOH | |
| Molar mass | 221.39 g·mol−1 |
| Appearance | yellow needles |
| Density | 7.44 g/cm3 |
| Melting point | decomposes at 139°C |
| 34.3 g/(100 g) at 18°C | |
| Thermochemistry | |
Std molar entropy (S⦵298) |
88.0 J·mol−1·K−1 |
Std enthalpy of formation (ΔfH⦵298) |
−238.9 kJ·mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Very toxic
Corrosive Dangerous for the environment |
| GHS labelling:[2] | |
   | |
| Danger | |
| H300, H330, H373, H411 | |
| P260, P264, P270, P271, P273, P284, P301+P310, P304+P340, P310, P314, P320, P330, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Thallium(I) hydroxide, also called thallous hydroxide, TlOH, is a hydroxide of thallium, with thallium in oxidation state +1.
Synthesis
Thallium(I) hydroxide is obtained from the decomposition of thallium(I) ethoxide in water.[3]
- CH3CH2OTl + H2O → TlOH + CH3CH2OH
This can also be done by direct reaction of thallium with ethanol and oxygen gas.
- 4 Tl + 2 CH3CH2OH + O2 → 2 CH3CH2OTl + 2 TlOH
Another method is the reaction between thallium(I) sulfate and barium hydroxide.
- Tl2SO4 + Ba(OH)2 → 2 TlOH + BaSO4
Properties
Thallous hydroxide is a strong base; it dissociates to the thallous ion, Tl+, except in strongly basic conditions. Tl+ resembles an alkali metal ion, such as Li+ or K+.
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 4–89, 5–16. ISBN 0-8493-0594-2.
- ↑ "Thallium hydroxide". pubchem.ncbi.nlm.nih.gov. Retrieved 12 December 2021.
- ↑ Brauer, Georg; Baudler, Marianne (1975). Handbuch der Präparativen Anorganischen Chemie, Band I. (3rd ed.). Stuttgart: Ferdinand Enke. p. 883. ISBN 3-432-02328-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
