 | |
| Names | |
|---|---|
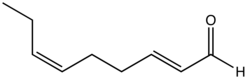
| Preferred IUPAC name
(2E,6Z)-Nona-2,6-dienal | |
| Other names
(E,Z)-2,6-Nonadienal Violet leaf aldehyde Cucumber aldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.345 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H14O | |
| Molar mass | 138.210 g·mol−1 |
| Appearance | Colorless oil |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H317 | |
| P261, P264, P272, P280, P302+P352, P321, P332+P313, P333+P313, P362, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
trans,cis-2,6-Nonadienal is an organic compound that is classified as a doubly unsaturated derivative of nonanal. The molecule consists of a α,β-unsaturated aldehyde with an isolated alkene group. The compound has attracted attention as the essence of cucumbers,[1][2] but it is also found in bread crust[3] and freshly cut watermelon.
Biosynthesis
Isotopic labeling has indicated that nonadienal is formed from α-linolenic acid.[4] Such reactions are typically catalyzed by hydroperoxide lyases.
See also
- 2-Nonenal - another potent odorant in cucumber
References
- ↑ Kula, Jozef; Sadowska, Halina (1993). "Unsaturated aliphatic C9-aldehydes as natural flavorants: (E,Z)-2,6-nonadienal". Perfumer & Flavorist. 18: 23–25.
- ↑ Schieberle, P.; Ofner, S.; Grosch, W. (January 1990). "Evaluation of Potent Odorants in Cucumbers (Cucumis sativus) and Muskmelons (Cucumis melo) by Aroma Extract Dilution Analysis". Journal of Food Science. 55 (1): 193–195. doi:10.1111/j.1365-2621.1990.tb06050.x.
- ↑ Cho, In Hee; Peterson, Devin G. (2010). "Chemistry of Bread Aroma: A Review". Food Science and Biotechnology. 19: 575–582. doi:10.1007/s10068-013-0240-4.
- ↑ Grosch, Werner; Schwarz, Jorg M. (May 1971). "Linoleic and linolenic acid as precursors of the cucumber flavor". Lipids. 6 (5): 351–352. doi:10.1007/BF02531828. S2CID 38868077.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.