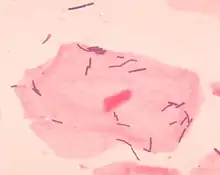
Vaginal flora, vaginal microbiota or vaginal microbiome are the microorganisms that colonize the vagina. They were discovered by the German gynecologist Albert Döderlein in 1892[1] and are part of the overall human flora. The amount and type of bacteria present have significant implications for an individual's overall health.[2] The primary colonizing bacteria of a healthy individual are of the genus Lactobacillus,[3] such as L. crispatus, and the lactic acid they produce is thought to protect against infection by pathogenic species.[4]
Lactobacilli
The primary colonizing bacteria of a healthy individual are of the genus Lactobacillus (90–95%), the most common being L. crispatus, L. iners, L. jensenii, and L. gasseri.[5] Since the first description of lactobacilli by Döderlein, lactobacilli have been generally considered the gatekeepers of the vaginal ecosystem. Lactobacilli have been shown to inhibit in vitro growth of pathogenic microorganisms, e.g. Bacteroides fragilis, Escherichia coli, Gardnerella vaginalis, Mobiluncus spp., Neisseria gonorrhoeae, Peptostreptococcus anaerobius, Prevotella bivia and Staphylococcus aureus. It is generally accepted that this is achieved mainly through the action of lactic acid.[6][7][8][9] Moreover, lactobacilli normally help to prevent long-term colonization of the vagina by adhering to vaginal epithelial cells. This usually reduces pathogens from infecting to the vaginal epithelium.[10]
Next to lactic acid production and competition for adherence, other antagonistic mechanisms include hydrogen peroxide (a broad-spectrum antimicrobial) and bacteriocins (target-specific antimicrobials) production.[11][12]
pH and lactic acid
Low pH is generally accepted to be the main mechanism controlling the composition of the vaginal microflora. Although the lactic acid produced by lactobacilli contributes to the vaginal acidity, it is still not proven to be the primary source of low vaginal pH, but the fact remains that most lactobacilli thrive best at a pH < 3.5 .[13][14][15] Normal vaginal pH is considered to be under 4.5 with a range of 3.8 to 4.4.[5]
Hydrogen peroxide
Production of hydrogen peroxide (H2O2) is a well-known mechanism for bacterial antagonism,[16][17][18] inhibiting growth of microorganisms via direct interaction or via human myeloperoxidase.[19][20][21] Hydrogen peroxide-producing lactobacilli have been shown to inactivate HIV-1, herpes simplex virus type 2 (HSV-2), Trichomonas vaginalis, G. vaginalis, P. bivia and E. coli. O'Hanlon[12] and Baeten[22] found that 96% of Lactobacillus species from a healthy vaginal ecosystem produced H2O2 (L. jensenii and L. vaginalis produce the highest levels of H2O2),[11][23] whereas only 6% of the lactobacilli recovered from women with BV produced H2O2.[19] In agreement with this, L. iners, most frequently associated with disturbed vaginal microflora,[24][25] is a poor producer of H2O2.[26][27] Vaginal colonization by H2O2-producing lactobacilli has been associated with a decrease in the occurrence of bacterial vaginosis (BV).[28] However, more recently O'Hanlon et al.[29] demonstrated that cervicovaginal fluid and semen have a significant H2O2-blocking activity and they later [12] demonstrated that physiological concentrations of H2O2 below 100 μM fail to inactivate any of the 17 tested BV-associated bacteria, e.g. A. vaginae, G. vaginalis, Mobiluncus spp., P. bivia, P. corporis, Mycoplasma hominis, even in the presence of human myeloperoxidase, known to increase the microbicidal activity of H2O2.[12] Only supraphysiologic concentrations of exogenous H2O2 (0.34% w/v, 100 mM) were sufficient to inactivate BV-associated bacteria at which concentration it more potently inactivated vaginal lactobacilli (L. crispatus, L. gasseri, L. iners and L. jensenii). A concentration of 100 mM H2O2 is approximately 50-fold higher than lactobacilli are capable of producing even under optimal aerobic, low-antioxidant conditions, and approximately 5,000-fold higher than the estimated H2O2 concentration in vivo. Even more remarkable, the addition of only 1% vaginal fluid blocked the microbicidal activity of 1 M H2O2. Possible explanations may be that cervicovaginal fluid and semen contain proteins, glycoproteins, polysaccharides, lipids, and other molecules with the potential to react with and inactivate H2O2. In addition, the vagina is hypoxic most of the time, whereas lactobacilli require oxygen to produce hydrogen peroxide. It is also remarkable that catalase, which provides bacteria protection against toxic H2O2, is absent in lactobacilli,[19][30] and as such they would be unprotected against their own H2O2 production. In contrast, under optimal anaerobic growth conditions, physiological concentrations of lactic acid inactivated the BV-associated pathogens without affecting the vaginal lactobacilli.[12][29] In summary, although the hydrogen peroxide production of lactobacilli has been considered as an important antimicrobial component, contributing to the colonization resistance provided by lactobacilli,[11][31] and although there seems to be a link between H2O2-producing lactobacilli and normal vaginal microflora, recent data do not support this role for H2O2.[12][29]
Bacteriocins
Vaginal lactobacilli produce antimicrobial peptides, i.e. bacteriocins such as lactocin 160 and crispasin.[13] with inhibitory activity ranging from narrow (closely related Lactobacillus species) to broad (diverse groups of bacteria, including G. vaginalis and P. bivia),[8] and bacteriocin-like substances, with a broader spectrum of activity than bacteriocins (e.g. a heat-resistant peptide produced by Ligilactobacillus salivarius subsp. salivarius CRL 1328). Several studies have indicated that the activity of bacteriocins is favored by low pH.
The inhibitory substances produced by vaginal Lactobacillus is a primary factor in protecting the vaginal microbiota, with organic acids, bacteriocins, and hydrogen peroxide. These act synergistically against infection by pathogens. Not all Lactobacillus spp. and not all strains within one Lactobacillus species exhibit all 3 mechanisms.[13] Lactobacillus species differ in premenopausal women, i.e. L. crispatus, L. jensenii, L. iners, L. gasseri (and possibly L. vaginalis), as assessed through cultivation-dependent and cultivation-independent techniques.[24][25][26][32] Vaginal lactobacilli have been shown to display a pronounced vaginotropism, and their pili act as ligands for attachment to receptors of vaginal epithelial cells. The limited number of Lactobacillus spp. found in the human vagina is remarkable, which leads to the possibility that there are host factors that select for specific organisms, that these species have unusual characteristics that allow them to successfully colonize the vagina, or both .[33] However, the vaginotropism, does not only apply to this selected group of lactobacilli that stand for a healthy vagina, but also for the bacterial species associated with BV.[34] The microbiota detected in the human genital and gut econiche do not appear to grow outside their host and probably are likely to rely on the close contact between parents and their children for transmission,[34] e.g. mother to neonate transmission of genital microflora, most probably also with gut microflora homogenously distributed over the baby's body including skin, the oral cavity, nasopharynx, and feces.[35]
Other microbiota
.jpg.webp)
Healthy, normal vaginal microbiota that is dominated by lactobacilli may differ among some ethnic groups. Non-pathogenic vaginal species are part of the normal microbiota of some women.[24][36] Several studies have demonstrated that a significant proportion (7–33%) of healthy asymptomatic women (especially black and Hispanic women)[37] lack appreciable numbers of Lactobacillus species in the vagina,[33][38] and instead have a vaginal microbiota that consist of other lactic acid-producing bacteria, i.e. species from the genera Atopobium, Leptotrichia, Leuconostoc, Megasphaera, Pediococcus, Streptococcus and Weissella,[32][33][37] All ethnic populations have vaginal microflora communities containing lactic acid producing bacteria.[33][37] This implies that not all communities may be equally resilient, so that if the resilience of a vaginal community is low then transitory changes in the structure of these communities may occur more readily in response to disturbances of various kinds, including menses, sexual intercourse, douching and contraceptive practices. These differences in the structure and composition of microbial communities may underlie well-known differences in the susceptibility of women in these racial groups to BV and various vaginal infections.[37][39][40] Though vaginal microflora may be disturbed, and lactic acid is produced by other species,[41] vaginal pH and acid production establish normal vaginal microbiota. The pH further decreases during pregnancy.[42]
Other vaginal bacterial species
Other bacterial species are frequently found in the vagina, such as the Gram positive cocci: Atopobium vaginae, Peptostreptococcus spp., Staphylococcus spp., Streptococcus spp., and Bacteroides spp., Fusobacterium spp., Gardnerella vaginalis, Mobiluncus, Prevotella spp., and Gram-negative enteric organisms, such as Escherichia coli.[24][25] Mycoplasma and Ureaplasma are frequently found in the vagina. Some of the obligate and facultative anaerobic bacteria are associated with BV.[38]
Pregnancy
The effect of tampon use on vaginal flora is debated, but application of tampons appears not to significantly modify the balance of bacterial presence.[43] Pregnancy alters the microbiota with a reduction in species/genus diversity.[44]
Disease prevention

A healthy vaginal microbiome aids in the prevention of bacterial vaginosis, yeast infections and other possible problems by maintaining an acidic pH (< 4.5) that is unfavourable for the growth of common pathogens, such as Gardnerella vaginalis. The lactobacilli present in a healthy vaginal microbiome also occupy the ecological niche that would otherwise be available for exploitation by pathogenic organisms. However, harmful bacteria or an imbalance in bacteria can lead to infection.[45]
Bacterial vaginosis is associated with the presence of Gardnerella vaginalis and Peptostreptococcus anaerobius[46] and a decrease in the number of Lactobacillus species that comprise the healthy vaginal microbiota.[44][47][48][49]
Research
Investigations have found that the presence of lacto-bacillus dominated bacteria in the vagina is associated with a lower incidence of sexually transmitted infections.[50][51]
See also
References
- ↑ David M (April 2006). "Albert und Gustav Döderlein – ein kritischer Blick auf zwei besondere Lebensläufe deutscher Ordinarien" [Albert and Gustav Döderlein -- a critical view to the biographies of two German professors]. Zentralblatt für Gynäkologie (in German). 128 (2): 56–9. doi:10.1055/s-2006-921412. PMID 16673245. S2CID 77926816.
- ↑ D'Ippolito S, Di Nicuolo F, Pontecorvi A, Gratta M, Scambia G, Di Simone N (December 2018). "Endometrial microbes and microbiome: Recent insights on the inflammatory and immune "players" of the human endometrium". American Journal of Reproductive Immunology. 80 (6): e13065. doi:10.1111/aji.13065. PMID 30375712. S2CID 53114757.
- ↑ Vásquez A, Jakobsson T, Ahrné S, Forsum U, Molin G (August 2002). "Vaginal lactobacillus flora of healthy Swedish women". Journal of Clinical Microbiology. 40 (8): 2746–9. doi:10.1128/JCM.40.8.2746-2749.2002. PMC 120688. PMID 12149323.
- ↑ Witkin SS, Linhares IM, Giraldo P (June 2007). "Bacterial flora of the female genital tract: function and immune regulation". Best Practice & Research. Clinical Obstetrics & Gynaecology. 21 (3): 347–54. doi:10.1016/j.bpobgyn.2006.12.004. PMID 17215167.
- 1 2 Tidbury, Fiona Damaris; Langhart, Anita; Weidlinger, Susanna; Stute, Petra (2020-10-06). "Non-antibiotic treatment of bacterial vaginosis—a systematic review". Archives of Gynecology and Obstetrics. Springer Science and Business Media LLC. 303 (1): 37–45. doi:10.1007/s00404-020-05821-x. ISSN 0932-0067. PMID 33025086. S2CID 222149236.
- ↑ Graver MA, Wade JJ (February 2011). "The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth". Annals of Clinical Microbiology and Antimicrobials. 10: 8. doi:10.1186/1476-0711-10-8. PMC 3045876. PMID 21329492.
- ↑ Matu MN, Orinda GO, Njagi EN, Cohen CR, Bukusi EA (June 2010). "In vitro inhibitory activity of human vaginal lactobacilli against pathogenic bacteria associated with bacterial vaginosis in Kenyan women". Anaerobe. 16 (3): 210–5. doi:10.1016/j.anaerobe.2009.11.002. PMID 19925874.
- 1 2 Skarin A, Sylwan J (December 1986). "Vaginal lactobacilli inhibiting growth of Gardnerella vaginalis, Mobiluncus and other bacterial species cultured from vaginal content of women with bacterial vaginosis". Acta Pathologica et Microbiologica Scandinavica, Section B. 94 (6): 399–403. doi:10.1111/j.1699-0463.1986.tb03074.x. PMID 3494379.
- ↑ Strus, M.; Malinowska, M.; Heczko, P.B. (2002). "In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis". J. Reprod. Med. 47: 41–46. PMID 11838310.
- ↑ Boris S, Barbés C (April 2000). "Role played by lactobacilli in controlling the population of vaginal pathogens". Microbes and Infection. 2 (5): 543–6. doi:10.1016/s1286-4579(00)00313-0. PMID 10865199.
- 1 2 3 Martín R, Suárez JE (January 2010). "Biosynthesis and degradation of H2O2 by vaginal lactobacilli". Applied and Environmental Microbiology. 76 (2): 400–5. Bibcode:2010ApEnM..76..400M. doi:10.1128/AEM.01631-09. PMC 2805228. PMID 19948869.
- 1 2 3 4 5 6 O'Hanlon DE, Moench TR, Cone RA (July 2011). "In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide". BMC Infectious Diseases. 11: 200. doi:10.1186/1471-2334-11-200. PMC 3161885. PMID 21771337.
- 1 2 3 Aroutcheva A, Gariti D, Simon M, Shott S, Faro J, Simoes JA, et al. (August 2001). "Defense factors of vaginal lactobacilli". American Journal of Obstetrics and Gynecology. 185 (2): 375–9. doi:10.1067/mob.2001.115867. PMID 11518895.
- ↑ Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS (February 2011). "Contemporary perspectives on vaginal pH and lactobacilli". American Journal of Obstetrics and Gynecology. 204 (2): 120.e1–5. doi:10.1016/j.ajog.2010.07.010. PMID 20832044.
- ↑ Redondo-Lopez V, Cook RL, Sobel JD (1990). "Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora". Reviews of Infectious Diseases. 12 (5): 856–72. doi:10.1093/clinids/12.5.856. PMID 2237129.
- ↑ Dahiya RS, Speck ML (October 1968). "Hydrogen peroxide formation by lactobacilli and its effect on Staphylococcus aureus". Journal of Dairy Science. 51 (10): 1568–72. doi:10.3168/jds.s0022-0302(68)87232-7. PMID 5682478.
- ↑ Thompson R, Johnson A (1951). "The inhibitory action of saliva on the diphtheria bacillus: hydrogen peroxide, the inhibitory agent produced by salivary streptococci". The Journal of Infectious Diseases. 88 (1): 81–5. doi:10.1093/infdis/88.1.81. PMID 14803753.
- ↑ Wheater DM, Hirsch A, Mattick AT (October 1952). "Possible identity of lactobacillin with hydrogen peroxide produced by lactobacilli". Nature. 170 (4328): 623–4. Bibcode:1952Natur.170..623W. doi:10.1038/170623a0. PMID 13002389. S2CID 4169333.
- 1 2 3 Eschenbach DA, Davick PR, Williams BL, Klebanoff SJ, Young-Smith K, Critchlow CM, Holmes KK (February 1989). "Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis". Journal of Clinical Microbiology. 27 (2): 251–6. doi:10.1128/JCM.27.2.251-256.1989. PMC 267286. PMID 2915019.
- ↑ Hillier SL, Krohn MA, Klebanoff SJ, Eschenbach DA (March 1992). "The relationship of hydrogen peroxide-producing lactobacilli to bacterial vaginosis and genital microflora in pregnant women". Obstetrics and Gynecology. 79 (3): 369–73. doi:10.1097/00006250-199203000-00008. PMID 1738516.
- ↑ Klebanoff SJ, Smith DC (1970). "Peroxidase-mediated antimicrobial activity of rat uterine fluid". Gynecologic Investigation. 1 (1): 21–30. doi:10.1159/000301903. PMID 4320447.
- ↑ Baeten JM, Hassan WM, Chohan V, Richardson BA, Mandaliya K, Ndinya-Achola JO, et al. (September 2009). "Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women". Sexually Transmitted Infections. 85 (5): 348–53. doi:10.1136/sti.2008.035451. PMC 2837477. PMID 19329442.
- ↑ Wilks M, Wiggins R, Whiley A, Hennessy E, Warwick S, Porter H, et al. (February 2004). "Identification and H(2)O(2) production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome". Journal of Clinical Microbiology. 42 (2): 713–7. doi:10.1128/jcm.42.2.713-717.2004. PMC 344438. PMID 14766841.
- 1 2 3 4 Verhelst R, Verstraelen H, Claeys G, Verschraegen G, Van Simaey L, De Ganck C, et al. (October 2005). "Comparison between Gram stain and culture for the characterization of vaginal microflora: definition of a distinct grade that resembles grade I microflora and revised categorization of grade I microflora". BMC Microbiology. 5: 61. doi:10.1186/1471-2180-5-61. PMC 1266370. PMID 16225680.
- 1 2 3 De Backer E, Verhelst R, Verstraelen H, Alqumber MA, Burton JP, Tagg JR, et al. (December 2007). "Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners". BMC Microbiology. 7: 115. doi:10.1186/1471-2180-7-115. PMC 2233628. PMID 18093311.
- 1 2 Antonio MA, Hawes SE, Hillier SL (December 1999). "The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species". The Journal of Infectious Diseases. 180 (6): 1950–6. doi:10.1086/315109. PMID 10558952.
- ↑ Antonio MA, Rabe LK, Hillier SL (August 2005). "Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis". The Journal of Infectious Diseases. 192 (3): 394–8. doi:10.1086/430926. PMID 15995952.
- ↑ Hawes SE, Hillier SL, Benedetti J, Stevens CE, Koutsky LA, Wolner-Hanssen P, Holmes KK (November 1996). "Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections". The Journal of Infectious Diseases. 174 (5): 1058–63. doi:10.1093/infdis/174.5.1058. PMID 8896509.
- 1 2 3 O'Hanlon DE, Lanier BR, Moench TR, Cone RA (May 2010). "Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli". BMC Infectious Diseases. 10: 120. doi:10.1186/1471-2334-10-120. PMC 2887447. PMID 20482854.
- ↑ Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM (July 1991). "Control of the microbial flora of the vagina by H2O2-generating lactobacilli". The Journal of Infectious Diseases. 164 (1): 94–100. doi:10.1093/infdis/164.1.94. PMID 1647428.
- ↑ Vallor AC, Antonio MA, Hawes SE, Hillier SL (December 2001). "Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production". The Journal of Infectious Diseases. 184 (11): 1431–6. doi:10.1086/324445. PMID 11709785.
- 1 2 Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. (March 2011). "Vaginal microbiome of reproductive-age women". Proceedings of the National Academy of Sciences of the United States of America. 108 Suppl 1 (Supplement 1): 4680–7. Bibcode:2011PNAS..108.4680R. doi:10.1073/pnas.1002611107. PMC 3063603. PMID 20534435.
- 1 2 3 4 Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ (August 2004). "Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods". Microbiology. 150 (Pt 8): 2565–2573. doi:10.1099/mic.0.26905-0. PMID 15289553.
- 1 2 Danielsson D, Teigen PK, Moi H (August 2011). "The genital econiche: focus on microbiota and bacterial vaginosis". Annals of the New York Academy of Sciences. 1230 (1): 48–58. Bibcode:2011NYASA1230...48D. doi:10.1111/j.1749-6632.2011.06041.x. PMID 21824165. S2CID 205936163.
- ↑ Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R (June 2010). "Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns". Proceedings of the National Academy of Sciences of the United States of America. 107 (26): 11971–5. Bibcode:2010PNAS..10711971D. doi:10.1073/pnas.1002601107. PMC 2900693. PMID 20566857.
- ↑ Santiago GL, Cools P, Verstraelen H, Trog M, Missine G, El Aila N, et al. (2011). "Longitudinal study of the dynamics of vaginal microflora during two consecutive menstrual cycles". PLOS ONE. 6 (11): e28180. Bibcode:2011PLoSO...628180L. doi:10.1371/journal.pone.0028180. PMC 3227645. PMID 22140538.
- 1 2 3 4 Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, et al. (June 2007). "Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women". The ISME Journal. 1 (2): 121–33. doi:10.1038/ismej.2007.12. PMID 18043622. S2CID 8143221.
- 1 2 Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, Reid G (August 2010). "Deep sequencing of the vaginal microbiota of women with HIV". PLOS ONE. 5 (8): e12078. Bibcode:2010PLoSO...512078H. doi:10.1371/journal.pone.0012078. PMC 2920804. PMID 20711427.
- ↑ Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ (August 2010). "Births: final data for 2007". National Vital Statistics Reports. 58 (24): 1–85. PMID 21254725.
- ↑ Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, et al. (March 2003). "Can known risk factors explain racial differences in the occurrence of bacterial vaginosis?". Journal of the National Medical Association. 95 (3): 201–12. PMC 2594421. PMID 12749680.
- ↑ Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT (January 2012). "A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility". AIDS Research and Human Retroviruses. 28 (1): 76–81. doi:10.1089/aid.2011.0071. PMC 3251838. PMID 21595610.
- ↑ Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. (December 1995). "Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group". The New England Journal of Medicine. 333 (26): 1737–42. doi:10.1056/nejm199512283332604. PMID 7491137.
- ↑ Briancesco R, Paduano S, Semproni M, Bonadonna L (September 2018). "A study on the microbial quality of sealed products for feminine hygiene". Journal of Preventive Medicine and Hygiene. 59 (3): E226–E229. doi:10.15167/2421-4248/JPMH2018.59.3.920. PMC 6196378. PMID 30397679.
- 1 2 Sharma H, Tal R, Clark NA, Segars JH (January 2014). "Microbiota and pelvic inflammatory disease". Seminars in Reproductive Medicine. 32 (1): 43–9. doi:10.1055/s-0033-1361822. PMC 4148456. PMID 24390920.
- ↑ Lewis, Felicia M. T.; Bernstein, Kyle T.; Aral, Sevgi O. (April 2017). "Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases". Obstetrics and Gynecology. 129 (4): 643–654. doi:10.1097/AOG.0000000000001932. ISSN 1873-233X. PMC 6743080. PMID 28277350.
- ↑ "Bacterial Vaginosis (BV): Condition Information". National Institute of Child Health and Human Development. 2013-05-21. Retrieved 3 March 2015.
- ↑ Nardis C, Mosca L, Mastromarino P (September 2013). "Vaginal microbiota and viral sexually transmitted diseases". Annali di Igiene. 25 (5): 443–56. doi:10.7416/ai.2013.1946. PMID 24048183.
- ↑ "What are the symptoms of bacterial vaginosis?". National Institute of Child Health and Human Development. 2013-05-21. Retrieved 22 May 2016.
- ↑ Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. (March 2011). "Vaginal microbiome of reproductive-age women". Proceedings of the National Academy of Sciences of the United States of America. 108 (Suppl. 1): 4680–7. Bibcode:2011PNAS..108.4680R. doi:10.1073/pnas.1002611107. PMC 3063603. PMID 20534435.
- ↑ Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, et al. (October 2015). "Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota". mBio. 6 (5): e01084-15. doi:10.1128/mBio.01084-15. PMC 4611035. PMID 26443453.
- ↑ Anderson DJ, Marathe J, Pudney J (June 2014). "The structure of the human vaginal stratum corneum and its role in immune defense". American Journal of Reproductive Immunology. 71 (6): 618–23. doi:10.1111/aji.12230. PMC 4024347. PMID 24661416.
External links
 Media related to Vaginal flora at Wikimedia Commons
Media related to Vaginal flora at Wikimedia Commons- Döderlein's bacteria