Arsenic in the solid state can be found as gray, black, or yellow allotropes. These various forms feature diverse structural motifs, with yellow arsenic enabling the widest range of reactivity. In particular, reaction of yellow arsenic with main group and transition metal elements results in compounds with wide-ranging structural motifs, with butterfly, sandwich and realgar-type moieties featuring most prominently.
Gray arsenic

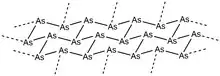
Gray arsenic or metallic arsenic is the most stable allotrope of the element at room temperature, and as such is its most common form.[1] This soft, brittle allotrope of arsenic has a steel grey, metallic color, and is a good conductor.[2] The rhombohedral form of this allotrope is analogous to the phosphorus allotrope black phosphorus. In its α-form, As6 rings in chair confirmations are condensed into packed layers lying perpendicular to the crystallographic c axis. Within each layer, the vicinal As-As bond distances are 2.517 Å, while the layer-to-layer As-As bond distances are 3.120 Å. The overall structure displays a distorted octahedral geometry, resulting in the largely metallic properties of this allotrope. Upon sublimation at 616 °C, the gas phase arsenic molecules lose this packing arrangement and form small clusters of As4, As2, and As, though As4 is by far the most abundant in this phase.[1] If these vapors are condensed swiftly onto a cold surface (<200 K), solid yellow arsenic (As4) results due to the lack of energy required to form the rhombohedral gray arsenic lattice. Conversely, condensation of arsenic vapors onto a heated surface generates amorphous black arsenic. The crystalline form of black arsenic can also be isolated, and the amorphous form can be annealed to return to the metallic gray arsenic form. Yellow arsenic can also be returned to the gray allotrope in a facile manner through application of light or by returning the molecule to room temperature.[1]
Reactivity
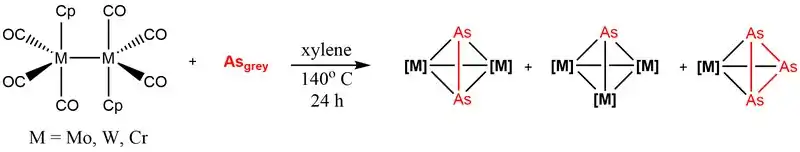
Relatively few in-situ reactions have been reported involving gray arsenic due to its low solubility, although it reacts in air to form gaseous As2O3 . Two examples of the reactivity of gray arsenic towards transition metals are known.[3][4] In these reactions, cyclopentadienyl complexes of molybdenum, tungsten and chromium proceed via loss of carbon monoxide to react with gray arsenic and form mono-, di-, and triarsenic compounds.
Black arsenic
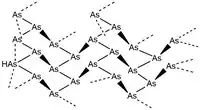
Black, or amorphous arsenic (chemical formula Asn) is synthesized first through the sublimation of gray arsenic followed by condensation onto a heated surface. This structure is thought to be the arsenic analogue of red phosphorus. The structure of black arsenic in its crystalline phase, while not synthesized in its pure form, is by extension analogous to black phosphorus, and takes on an orthorhombic structure built from As6 rings. Black arsenic has as-yet been synthesized only in the presence of atomic impurities including mercury,[5] phosphorus, and oxygen, though a pure form of black arsenic was found in the Copiapó region of Chile. Mechanical exfoliation of the mineral found in Chilean caves, arsenolamprite, revealed a molecular structure with high in-phase anisotropy and potential as a semiconducting material.[6]
Yellow arsenic
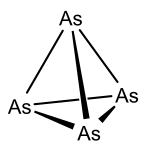
Rapid condensation of arsenic vapors on to a cold surface results in the formation of yellow arsenic (As4), consisting of four arsenic atoms arranged in a tetrahedral geometry analogous to white phosphorus. Though it is the only soluble form of arsenic known, yellow arsenic is metastable: at room temperature, or in the presence of light, the structure quickly decomposes to adopt the lower-energy configuration of gray arsenic. For this reason, extensive care is required to maintain yellow arsenic in a state suitable for reaction, including rigorous exclusion of light and maintenance of temperatures below -80 °C.[1] Yellow arsenic is the allotrope most suited for reactivity studies, due to its solubility (low, but comparatively large relative to the metallic allotrope) and molecular nature. In comparison to its lighter congener, phosphorus, the reactivity of arsenic is relatively underexplored. Research investigating reactions with arsenic are primarily concerned with the activation of main group and transition metal compounds; in the case of transition metal complexes, As4 has demonstrated competent reactivity across the d-block of the periodic table.
Reactivity towards main group compounds
The first activation of a main group compound by yellow arsenic was reported in 1992 by West and coworkers, involving the reaction of As4 with a disilene compound, tetramesityldisilene, to generate a mixture of compounds including a butterfly structural motif of bridging arsenic atoms.[7] Notably, the product mixture obtained in this reaction differs from the analogous reaction with P4 that produces the butterfly compound alone, highlighting that the reactivity of yellow arsenic and white phosphorus cannot be considered identical. The first organo-substituted As4 compound was produced by Scheer and coworkers in 2016 via reaction with the CpPEt radical.[8] Analogous to the butterfly compound obtained by the West group, the product obtained in this reaction featured a bridging As4 motif that reversibly returned As4 and the parent radical in the presence of light or heat. This characteristic makes the CpPEt2As4 complex a uniquely suitable "storage" molecule for yellow arsenic, as it is stable when stored at room temperature in the dark, but can release As4 in thermal or photochemical solutions.
Other reactions of main group compounds with yellow arsenic have been shown to involve units of arsenic with more than four atoms. In reaction with the silylene compound [PhC(NtBu)2SiN(SiMe3)2], an aggregation of As4 was observed to form a cage compound of ten arsenic atoms, including a seven-membered arsenic ring at its center.[9]
Reactivity towards transition metal compounds

Group 4 and 5 metals
Among the early (group 4 and 5) transition metal elements, few examples of arsenic activation has been reported to date. Carbon monoxide complexes of zirconium with derivatized cyclopentadienyl ligands were shown to react with yellow arsenic in boiling xylene to release CO and bind the As4 moiety in η1:1-fashion.[11] Trace amounts of a zirconium dimer bridged by a (μ,η2:2:1-As5)-moiety were also reported in this study, which described the complexes as possible reagents for As4 transfer. In group 5, arsenic activation has been more widely explored, with complexes of both niobium and tantalum known.[10][12] Investigation of the electron density topology in a phosphorus/arsenic/niobium-containing system demonstrated the unique η2-bonding configuration in these complexes, in which an arsenic-phosphorus double bond binds side-on to a niobium center.
Group 6 metals
Reactions of yellow arsenic with the group 6 transition metals largely proceed through thermolytic carbon monoxide elimination in chromium and molybdenum carbonyl complexes. Notable examples include the formation of triple-decker complexes [(CpRMo)2(μ,η6-As6)] and [{CpRCr}2(μ,η5-As5)] via reaction of the corresponding molybdenum and chromium dimers with yellow arsenic.[14][13] These remarkable structures feature three planar-rings arranged in parallel fashion to result in an idealized D5h point group for the chromium complex. Both of these reactions necessitate harsh reaction conditions like boiling xylene to overcome the high barriers to activation of As4. Conversely, utilization of more sterically demanding ligands on the metal center enabled reactions in milder conditions with molybdenum and chromium. Cummins' Mo(N(tBu)Ar)3 catalyst, also known to split the N-N triple bond in dinitrogen, reacts with yellow arsenic to form a terminal arsenic moiety triple-bonded to the metal center - one of only several compounds known to contain a terminal arsenic atom.[15] Complexes with metal-metal multiple bonds also enable mild As4 activation parameter. A chromium-chromium quintuply-bonded species reported by Kempe reacts with yellow arsenic to form a crown complex in which the four arsenic atoms form an approximately tetrahedral structure, with each chromium atom bonding to three arsenic atoms.[16]
Group 8 and 9 metals
The metals of groups 8 and 9 feature the most extensive library of reactivity with yellow arsenic documented in the scientific literature, with particular focus on reactions of iron and cobalt complexes with As4. Much like the chromium and molybdenum sandwich complexes, (CpRFe(CO)2]2 complexes of iron react with yellow arsenic to produce analogous bimetallic products featuring "triple-decker" geometry. These reports also detail the isolation of a key intermediate, pentaarsaferrocene ([CpRFe(μ5-As5)]).[18] This intermediate, isolobal to ferrocene, replaces one of the cyclopentadienyl ligands with a cyclic As5 ligand that features As-As bond lengths of 2.312 Å (in line with delocalized As-As double bonds). This "sandwich-forming" reactivity can be meaningfully tuned by introducing bulkier ligands. Modifying the cyclopentadienyl groups with much bulkier derivatives produces a vastly different set of products. First, a butterfly complex with a central As4 unit is formed. Irradiation with light leads to further CO elimination and the formation of a bridged butterfly complex, which then rearranges into a unique complex featuring a central As8 moiety. This ligand, formally tetraanionic, forms an eight-membered ring bridging four iron atoms in total.[17]
Much of the same reactivity, including formation of butterfly and sandwich compounds, has been described for cobalt complexes in the presence of yellow arsenic. Beyond these compounds, the history of reactivity of cobalt and yellow arsenic dates back to 1978, when Sacconi and coworkers reported the reaction of cobalt tetrafluoroborate and yellow arsenic in the presence of 1,1,1-tris(diphenylphosphinomethyl)ethane. The resulting complex features a cyclic As3 moiety bridging two cobalt centers, of which the former is assigned formally as a 3π-electron system.[19] The reaction of [Cp*Co(CO)]2 dimer with yellow arsenic was shown by Scherer et al. to produce a wide variety of isolable products, featuring a mixture of linking arsenic moieties including cyclobutane-like and butterfly type complexes.[20] Analogous reactions with rhodium complexes are also known.[21]
Group 10 and 11 metals
Among the group 10 and 11 elements, nickel and copper feature most prominently in literature reactions with yellow arsenic. Nickel tetrafluoroborate salts react analogously to cobalt complexes in the presence of triphos to form a sandwich structure with a central cyclic As3 moiety. Much like iron, the reaction of nickel cyclopentadienyl carbonyl complexes with As4 yields a variety of bi- and multi-metallic products depending on the size of the attending ligands, though the nature and geometric structure of these compounds differ from those observed with iron.[19] These include trimers with bridging As4 and As5 moieties in cubane structural arrangements when smaller Cp ligands are employed, and distorted hexagonal prism complexes with two nickel fragments and four arsenic atoms when bulkier Cp groups are introduced.
The reaction of the copper complex [L2Cu(NCMe)] (L2 = [{N(C6H3iPr2-2,6)C(Me)}2CH]) with yellow arsenic yields the As4-bridged dimer [{L2Cu}2- (μ,η2:2-As4)].[22] The four-atom arsenic moiety in this complex was deemed to be "intact" yellow arsenic through the use of density functional theory calculations determining the change in bond critical points between the free and bound arsenic molecules. Specifically, only a small shift was observed in the bond critical points between arsenic atoms involved in binding to copper; the remaining bond critical points were very similar to free yellow arsenic.
See also
References
- 1 2 3 4 5 6 7 Seidl, Michael; Balázs, Gábor; Scheer, Manfred (2019-03-22). "The Chemistry of Yellow Arsenic". Chemical Reviews. 119 (14): 8406–8434. doi:10.1021/acs.chemrev.8b00713. ISSN 0009-2665. PMID 30900440. S2CID 85448636.
- ↑ "Allotrope : Arsenic". dirkncl.github.io. Retrieved 2020-11-01.
- 1 2 Ziegler, M.L. (1988). "Darstellung und Charakterisierung von Tetrahedranen des Typs Cp3M3As(CO)6 und Cp2M2As2(CO)4 (Cp = C5H5, M = Mo, W) sowie von Derivaten dieser Tetrahedrane". Chemische Berichte. 121 (1). doi:10.1002/cber.v121:1. ISSN 0009-2940.
- 1 2 Goh, Lai Yoong.; Wong, Richard C. S.; Yip, Wai Hing.; Mak, Thomas C. W. (1991). "Synthesis and thermolysis of di- and triarsenic complexes of chromium. Crystal structure of [CpCr(CO)2]2As2". Organometallics. 10 (4): 875–879. doi:10.1021/om00050a015. ISSN 0276-7333.
- ↑ Antonatos, Nikolas; Luxa, Jan; Sturala, Jiri; Sofer, Zdenek (2020). "Black Arsenic: A New Synthetic Method by Catalytic Crystallization of Arsenic Glass". Nanoscale. 12 (9): 5397–5401. doi:10.1039/C9NR09627B. ISSN 2040-3372. PMID 31894222. S2CID 209544160.
- ↑ Chen, Yabin; Chen, Chaoyu; Kealhofer, Robert; Liu, Huili; Yuan, Zhiquan; Jiang, Lili; Suh, Joonki; Park, Joonsuk; Ko, Changhyun; Choe, Hwan Sung; Avila, José (2018). "Black Arsenic: A Layered Semiconductor with Extreme In-Plane Anisotropy". Advanced Materials. 30 (30): 1800754. arXiv:1805.00418. doi:10.1002/adma.201800754. ISSN 1521-4095. PMID 29893020.
- ↑ Tan, Robin P.; Comerlato, Nadia M.; Powell, Douglas R.; West, Robert (1992). "The Reaction of Tetramesityldisilene with As4: Synthesis and Structure of a Novel Arsenic–Silicon Tricyclic Ring System". Angewandte Chemie International Edition in English. 31 (9): 1217–1218. doi:10.1002/anie.199212171. ISSN 1521-3773.
- 1 2 Heinl, Sebastian; Balázs, Gábor; Stauber, Andreas; Scheer, Manfred (2016-11-15). "CpPEt2As4-An Organic-Substituted As4Butterfly Compound". Angewandte Chemie International Edition. 55 (50): 15524–15527. doi:10.1002/anie.201608478. ISSN 1433-7851. PMID 27862725.
- 1 2 Seitz, Andreas E.; Eckhardt, Maria; Sen, Sakya S.; Erlebach, Andreas; Peresypkina, Eugenia V.; Roesky, Herbert W.; Sierka, Marek; Scheer, Manfred (2017). "Different Reactivity of As4 towards Disilenes and Silylenes". Angewandte Chemie International Edition. 56 (23): 6655–6659. doi:10.1002/anie.201701740. ISSN 1521-3773. PMID 28471032.
- 1 2 Spinney, Heather A.; Piro, Nicholas A.; Cummins, Christopher C. (2009-11-11). "Triple-Bond Reactivity of an AsP Complex Intermediate: Synthesis Stemming from Molecular Arsenic, As4". Journal of the American Chemical Society. 131 (44): 16233–16243. doi:10.1021/ja906550h. hdl:1721.1/65118. ISSN 0002-7863. PMID 19842699.
- ↑ Schmidt, Monika; Seitz, Andreas E.; Eckhardt, Maria; Balázs, Gábor; Peresypkina, Eugenia V.; Virovets, Alexander V.; Riedlberger, Felix; Bodensteiner, Michael; Zolnhofer, Eva M.; Meyer, Karsten; Scheer, Manfred (2017-09-27). "Transfer Reagent for Bonding Isomers of Iron Complexes". Journal of the American Chemical Society. 139 (40): 13981–13984. doi:10.1021/jacs.7b07354. ISSN 0002-7863. PMID 28933848.
- ↑ Scherer, Otto J.; Vondung, Jürgen; Wolmershäuser, Gotthelf (1989). "Tetraphosphacyclobutadiene as Complex Ligand". Angewandte Chemie International Edition in English. 28 (10): 1355–1357. doi:10.1002/anie.198913551. ISSN 1521-3773.
- 1 2 Scherer, Otto J.; Wiedemann, Wolfgang; Wolmershäuser, Gotthelf (1990). "Chrom-Komplexe mitcyclo-Asx-Liganden". Chemische Berichte (in German). 123 (1): 3–6. doi:10.1002/cber.19901230102.
- 1 2 Scherer, O. J. (1989). "A Triple- Decker Sandwich Complex with an Unstrained Cyclic Pentaarsane Middle Layer". J. Organomet. Chem. 361: C11−C14. doi:10.1016/0022-328X(89)87363-2.
- ↑ Curley, John J.; Piro, Nicholas A.; Cummins, Christopher C. (2009-10-19). "A Terminal Molybdenum Arsenide Complex Synthesized from Yellow Arsenic". Inorganic Chemistry. 48 (20): 9599–9601. doi:10.1021/ic9016068. hdl:1721.1/64721. ISSN 0020-1669. PMID 19764796.
- ↑ Schwarzmaier, Christoph; Noor, Awal; Glatz, Germund; Zabel, Manfred; Timoshkin, Alexey Y.; Cossairt, Brandi M.; Cummins, Christopher C.; Kempe, Rhett; Scheer, Manfred (2011). "Formation of cyclo-E42− Units (E4=P4, As4, AsP3) by a Complex with a Cr—Cr Quintuple Bond". Angewandte Chemie International Edition. 50 (32): 7283–7286. doi:10.1002/anie.201102361. ISSN 1521-3773. PMID 21698734.
- 1 2 Schwarzmaier, Christoph; Timoshkin, Alexey Y.; Balázs, Gábor; Scheer, Manfred (2014). "Selective Formation and Unusual Reactivity of Tetraarsabicyclo[1.1.0]butane Complexes". Angewandte Chemie International Edition. 53 (34): 9077–9081. doi:10.1002/anie.201404653. ISSN 1521-3773. PMID 25123699.
- ↑ Scherer, O. J.; Blath, Christof; Wolmershäuser, Gotthelf (1990-05-01). "Ferrocene mit einem Pentaarsacyclopentadienyl-Liganden". Journal of Organometallic Chemistry (in German). 387 (2): C21–C24. doi:10.1016/0022-328X(90)80029-Y. ISSN 0022-328X.
- 1 2 Di Vaira, Massimo; Midollini, Stefano; Sacconi, Luigi (1979). "cyclo-Triphosphorus and cyclo-triarsenic as ligands in "double sandwich" complexes of cobalt and nickel". Journal of the American Chemical Society. 101 (7): 1757–1763. doi:10.1021/ja00501a019. ISSN 0002-7863.
- ↑ Scherer, Otto J.; Pfeiffer, Karl; Wolmershäuser, Gotthelf (1992-11-01). "Cobaltkomplexe mit As4-Liganden". Chemische Berichte. 125 (11): 2367–2372. doi:10.1002/cber.19921251107. ISSN 0009-2940.
- ↑ Scherer, Otto J.; Höbel, Bernd; Wolmershäuser, Gotthelf (1992). "Zweifach kantengeöffnetes P10-Dihydrofulvalen als 16-Elektronendonorligand". Angewandte Chemie. 104 (8): 1042–1043. doi:10.1002/ange.19921040811. ISSN 0044-8249.
- ↑ Spitzer, Fabian; Sierka, Marek; Latronico, Mario; Mastrorilli, Piero; Virovets, Alexander V.; Scheer, Manfred (2015). "Fixation and Release of Intact E4 Tetrahedra (E=P, As)". Angewandte Chemie International Edition. 54 (14): 4392–4396. doi:10.1002/anie.201411451. ISSN 1521-3773. PMID 25677593.