 | |
| Clinical data | |
|---|---|
| Trade names | Yupelri |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619009 |
| License data |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
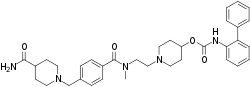
| Formula | C35H43N5O4 |
| Molar mass | 597.760 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Revefenacin, sold under the brand name Yupelri, is a medication for the treatment of chronic obstructive pulmonary disease (COPD). It was approved for use in the United States in 2018.[1] It was developed by Theravance Biopharma and is marketed by Mylan. Revefenacin is formulated as a solution that is nebulized and inhaled.[2]
Revefenacin is a bronchodilator that exerts its effect as a long-acting muscarinic antagonist.[3]
References
- ↑ "Theravance Biopharma and Mylan Receive FDA Approval for Yupelri (revefenacin) in Adults with Chronic Obstructive Pulmonary Disease" (Press release). Mylan. 9 November 2018. Archived from the original on 15 September 2019. Retrieved 17 January 2019.
- ↑ Heo YA (January 2019). "Revefenacin: First Global Approval". Drugs. 79 (1): 85–91. doi:10.1007/s40265-018-1036-x. PMC 6445810. PMID 30560478.
- ↑ Donohue JF, Kerwin E, Barnes CN, Moran EJ, Haumann B, Crater GD (May 2020). "Efficacy of revefenacin, a long-acting muscarinic antagonist for nebulized therapy, in patients with markers of more severe COPD: a post hoc subgroup analysis". BMC Pulmonary Medicine. 20 (1): 134. doi:10.1186/s12890-020-1156-4. PMC 7216337. PMID 32393215.
External links
- "Revefenacin". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.