溴酸盐
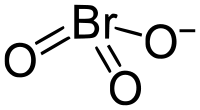
溴酸盐是溴酸形成的盐类,含有三角锥型的溴酸根离子—BrO3−,其中溴的氧化态为+5。溴酸盐(如溴酸钡、溴酸银等)都可溶于水,一些碱式盐除外。[1]
| 溴酸盐 | |
|---|---|
 | |
 | |
| 识别 | |
| CAS号 | 15541-45-4 |
| PubChem | 84979 |
| ChemSpider | 76658 |
| SMILES |
|
| ChEBI | 29223 |
| 性质 | |
| 化学式 | BrO3− |
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |
- Br− + O3 → BrO3−
实验室中,溴酸盐可通过将溴单质溶于氢氧化物浓溶液中制得,如:
- Br2 + 2OH− → Br− + BrO− + H2O
- 3BrO− → BrO3−+ 2Br−
参考资料
- Prescott, A.B.; Johnson, O.C. . D. Van Nostrand Company. 1901: 349 [2021-11-27]. (原始内容存档于2021-11-27).
- . International Agency for Research on Cancer: Summaries and Evaluations. Canadian Centre for Occupational Health and Safety. [2008-03-09]. (原始内容存档于2017-06-30).
- Kurokawa, Yuji. . Environmental Health Perspectives. July 1990, 87: 309–335 [2008-03-09]. doi:10.2307/3431039.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.