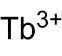草酸铽
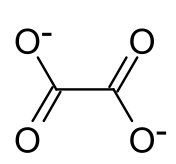
草酸铽是铽的草酸盐,化学式为Tb2(C2O4)3,其十水合物可由氯化铽和草酸在水溶液中反应得到。[1]它的十水合物受热逐步失水,并得到无水物,继续加热得到七氧化四铽,[2]隔绝空气分解生成三氧化二铽,分解的气体产物是一氧化碳和二氧化碳。[1]它和盐酸反应得到H[Tb(C2O4)2]·6H2O。[3]
| 草酸铽 | |||
|---|---|---|---|
 | |||
| |||
| |||
| 识别 | |||
| CAS号 | 996-33-8 | ||
| SMILES |
| ||
| 性质 | |||
| 化学式 | Tb2(C2O4)3 | ||
| 外观 | 白色固体,紫外线下发出绿光(十水)[1] | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
参考文献
- Dinu Alexander, Kukku Thomas, S. Sisira, P.R. Biju, N.V. Unnikrishnan, M.A. Ittyachen, Cyriac Joseph. . Dyes and Pigments. 2018-01, 148: 386–393 [2020-10-11]. doi:10.1016/j.dyepig.2017.09.029. (原始内容存档于2018-06-30) (英语).
- Wendlandt, W. W. . Analytical Chemistry. 1959, 31 (3): 408–410. ISSN 0003-2700. doi:10.1021/ac60147a024.
- Moebius, R.; Matthes, F. The exchange of oxalate ions for chloride ions of the oxalate hydrates of the rare earths and yttrium. Zeitschrift fuer Chemie, 1964. 4 (6): 234-235. ISSN: 0044-2402.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.