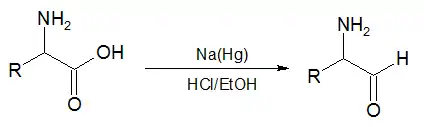赤堀氨基酸反应
赤堀氨基酸反应(Akabori amino acid reaction)有两种:

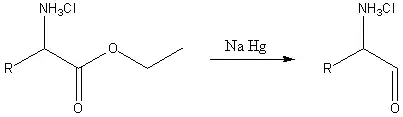
There are several Akabori amino acid reactions, which are named after Shirō Akabori (jap. 赤堀 四郎) (1900–1992), a Japanese chemist.
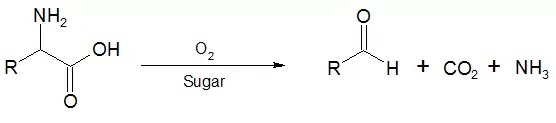
In the first reaction, an α-amino acid is oxidised and undergoes decarboxylation to give an aldehyde at the former α position by heating with oxygen in the presence of a reducing sugar.[1][2] This reaction is useful for preparing dichlorophthalimido derivatives of peptides for mass spectral analysis.[3]
In the second reaction, an α-amino acid, or an ester of it, is reduced by sodium amalgam and ethanolic HCl to give an α-amino aldehyde.[4][5] This process is conceptually similar to the Bouveault–Blanc reduction[6][7][8] except that it stops at the aldehyde stage rather than reducing the ester all the way to two alcohols.
参见
参考资料
- S. Akabori. . J. Chetn. Soc. Japan, Pure Chem. Sect. 1931, 52: 606–810.
- S. Akabori. . Chem. Ber. 1933, 66 (2): 143. doi:10.1002/cber.19330660213 (德语).
- . Comprehensive Organic Name Reactions and Reagents. 2010, 8: 29–32. doi:10.1002/9780470638859.conrr008.
- A. Lawson, H.V. Motley. . J. Chem. Soc. 1955: 1695. doi:10.1039/jr9550001695.
- A. Lawson. . J. Chem. Soc. 1956: 307. doi:10.1039/jr9560000307.
- Bouveault, Louis; Blanc, Gustave Louis. [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. 1903, 136: 1676–1678 [2019-03-08]. (原始内容存档于2019-03-27) (法语).
- Bouveault, Louis; Blanc, Gustave Louis. [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. 1903, 137: 60–62 (法语).
- Bouveault, Louis; Blanc, Gustave Louis. [Transforming saturated monobasic acids into the corresponding primary alcohols]. Bull. Soc. Chim. Fr. 1904, 31: 666–672 [2019-03-08]. (原始内容存档于2019-11-15) (法语).
参考资料
- S. Akabori, J. Chem. Soc. Japan, 52, 606(1931); Ber, 66, 143,151(1933); J. Chem. Soc. Japan, 64, 608(1943)
- E. Takagi, et al., J. Pharm. Soc. Japan, 71, 648(1951); 72,812(1952)
- A. Lawson, H.V. Motley, J. Chem. Soc., 1955, 1695
- A. Lawson, J. Chem. Soc. 1956, 307
- Reference Source(页面存档备份,存于)