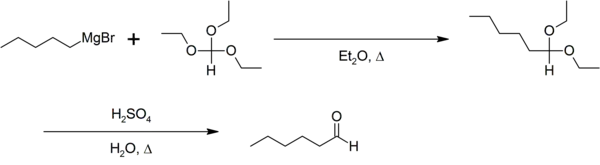Bodroux–Chichibabin醛合成
波尔多-齐齐巴宾醛合成(Bodroux-Chichibabin aldehyde synthesis)[1][2][3][4][5]
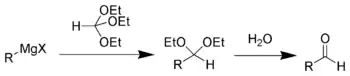
格氏试剂与原甲酸三乙酯反应生成缩醛,缩醛经过水解得到比格氏试剂多一个碳的醛类。
参考资料
- Bodroux, F. Compt. Rend. 1904, 138: 92. 缺少或
|title=为空 (帮助) - Tschitschibabin, A. E. . Ber. 1904, 37: 186. doi:10.1002/cber.19040370133.
- Tschitschibabin, A. E. . Ber. 1904, 37: 850. doi:10.1002/cber.190403701140.
- Smith, L. I.; Bayliss, M. . J. Org. Chem. 1941, 6: 437. doi:10.1021/jo01203a009.
- Smith, L. I.; Nichols, J. . J. Org. Chem. 1941, 6: 489. doi:10.1021/jo01204a003.
- G. Bryant Bachman (1943). "n-Hexaldehyde". Org. Synth.; Coll. Vol. 2: 323.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.