福山还原反应
Fukuyama还原反应(福山还原反应,Fukuyama reduction),由日本化学家福山透首先发现。
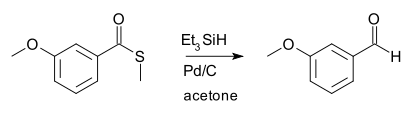
硫酯用氢硅烷(silyl hydride)在催化量钯存在下还原,得到醛。[1] 福山透最早所用的氢硅烷为三乙基硅烷,催化剂为钯碳:
此反应用于将羧酸分步还原为醛。
反应机理
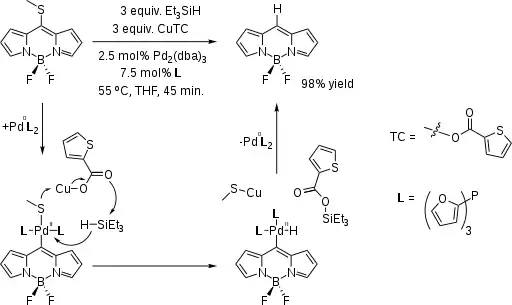
反应的催化循环如下:
- 氧化加成:
- R-C(O)-SR + pd(0) → RC(O)-Pd(II)-SR
- 配体交换:
- RC(O)-Pd(II)-SR + R3SiH → RC(O)-Pd(II)-H + R3Si-SR
- 还原消除:
- RC(O)-Pd(II)-H → RC(O)-H + Pd(0)
参见
参考资料
- Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+)-neothramycin A methyl ether Tohru Fukuyama, Shao Cheng Lin, Leping Li J. Am. Chem. Soc., 1990, 112 (19), pp 7050–7051 doi:10.1021/ja00175a043
- The Smallest and One of the Brightest. Efficient Preparation and Optical Description of the Parent Borondipyrromethene System. I. J. Arroyo, R. Hu, G. Merino, B. Z. Tang, E. Peña-Cabrera, J. Org. Chem. 2009, ASAP
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.