 | |
 | |
| Names | |
|---|---|
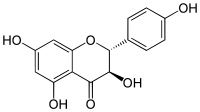
| IUPAC name
(2R,3R)-3,4′,5,7-Tetrahydroxyflavan-4-one | |
| Systematic IUPAC name
(2R,3R)-3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Aromadendrin Dihydrokaempferol Aromadendrol (+)-Aromadendrin (+)-Dihydrokaempferol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.213.374 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O6 | |
| Molar mass | 288.255 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Aromadendrin (aromodendrin or dihydrokaempferol) is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.[1]
Metabolism
The enzyme dihydrokaempferol 4-reductase uses cis-3,4-leucopelargonidin and NADP+ to produce (+)-aromadendrin, NADPH, and H+.
Glycosides
(2R,3R)-trans-Aromadendrin-7-O-beta-D-glucopyranoside-6′′-(4′′′-hydroxy-2′′′-methylene butanoate) is an acylated glucoside of aromadendrin isolated from the stem bark of Afzelia bella[2] (Fabaceae).
Phellamurin is the 8-prenyl 7-glucoside derivative of aromadendrin.
Chemistry
(+)-Leucopelargonidin, (2R,3S,4R)-3,4,5,7,4'-pentahydroxyflavan, can be synthesized from (+)-aromadendrin by sodium borohydride reduction.[3]
References
- ↑ V. I. Lutskii, A. S. Gromova and N. A. Tyukavkina (1971). "Aromadendrin, apigenin, and kaempferol from the wood of Pinus sibirica". Chemistry of Natural Compounds. 7 (2): 197–198. doi:10.1007/BF00568701.
- ↑ Binutu, OA; Cordell, GA (2001). "Constituents of Afzelia bella stem bark". Phytochemistry. 56 (8): 827–30. doi:10.1016/S0031-9422(01)00006-1. PMID 11324912.
- ↑ Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta. 163 (2): 191–196. doi:10.1007/BF00393505. PMID 24249337.
External links
 Media related to Aromadedrin at Wikimedia Commons
Media related to Aromadedrin at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.