 | |
| Names | |
|---|---|
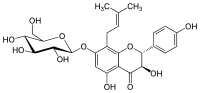
| IUPAC name
(2R,3R)-7-(β-D-Glucopyranosyloxy)-3,4′,5-trihydroxy-8-(3-methylbut-2-en-1-yl)flavan-4-one | |
| Systematic IUPAC name
(2R,3R)-3,5-Dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | C016043 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H30O11 | |
| Molar mass | 518.515 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phellamurin, a flavonoid, is the 7-O-β-D-glucopyranoside, 8-C-prenyl derivative of the flavan-on-ol Aromadendrin,[1] and may be seen as the 7-O-glucoside of noricaritin.[2] Being a flavanonol, it has two stereocenters on the C-ring, so four stereoisomers of phellamurin are possible.
It can be found in Commiphora africana[3] and in Phellodendron amurense.[4]
Related compounds
6"′-O-acetyl phellamurin is found in the leaves of Phellodendron japonicum.[5]
References
- ↑ "Phellamurin".
- ↑ Fortschritte der Chemie Organischer Naturstoffe. p. 17. ISBN 370918052X.
- ↑ Ma, Ji; Jones, Shannon H.; Hecht, Sidney M. (2005). "A Dihydroflavonol Glucoside from Commiphora a fricana that Mediates DNA Strand Scission". Journal of Natural Products. 68 (1): 115–117. doi:10.1021/np0400510. PMID 15679332.
- ↑ Honda, Keiichi; Hayashi, Nanao (1995). "A flavonoid glucoside, phellamurin, regulates differential oviposition on a rutaceous plant,Phellodendron amurense, by two sympatric swallowtail butterflies,Papilio protenor andP. Xuthus: The front line of a coevolutionary arms race?". Journal of Chemical Ecology. 21 (10): 1531–1539. doi:10.1007/BF02035150. PMID 24233681. S2CID 8258780.
- ↑ Chiu, C. Y.; Li, C. Y.; Chiu, C. C.; Niwa, M.; Kitanaka, S.; Damu, A. G.; Lee, E. J.; Wu, T. S. (2005). "Constituents of leaves of Phellodendron japonicum MAXIM. And their antioxidant activity". Chemical & Pharmaceutical Bulletin. 53 (9): 1118–1121. doi:10.1248/cpb.53.1118. PMID 16141579.
External links
 Media related to Phellamurin at Wikimedia Commons
Media related to Phellamurin at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.