 | |
| Names | |
|---|---|
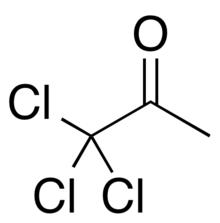
| Preferred IUPAC name
1,1,1-Trichloropropan-2-one | |
| Other names
1,1,1-Trichloroacetone 1,1,1-Trichloropropanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.149.432 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H3Cl3O | |
| Molar mass | 161.41 g·mol−1 |
| Appearance | Colourless liquid |
| Density | 1.475 g/cm3 |
| Boiling point | 134 °C (273 °F; 407 K)[1] |
| slightly soluble | |
| Solubility | Soluble in ethanol and diethyl ether |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 64 °C (147 °F; 337 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,1,1-Trichloroacetone is a chlorinated analogue of acetone with the chemical formula CH3COCCl3. It is a colourless liquid. 1,1,1-Trichloroacetone can be synthesised from chlorination of chloroacetone (1,1,3-trichloroacetone is formed as a by-product). An alternative synthesis involves the transfer of a trichloromethyl group from trichloroacetate onto acetyl chloride.[2]
See also
References
- ↑ "1,1,1-TRICHLOROACETONE CAS#: 918-00-3". m.chemicalbook.com.
- ↑ Taschner, Michael J. (2001). "Sodium Trichloroacetate". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rs113. ISBN 0471936235.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.