 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,1,3,3-Tetramethoxypropane | |
| Other names
1,1,3,3-Tetrakis(methyloxy)propane; Malonaldehyde, bis(dimethyl acetal) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ECHA InfoCard | 100.002.762 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H16O4 | |
| Molar mass | 164.201 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9895 g/cm3 |
| Boiling point | 183 °C (361 °F; 456 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
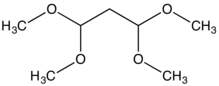
1,1,3,3-Tetramethoxypropane is an organic compound with the formula CH2(CH(OCH3)2)2. A colorless liquid, it is a protected form of malondialdehyde, a usefully reactive reagent that has poor storage properties.[1]
References
- ↑ V. Nair, C. L. O'Neil, P. G. Wang "Malondialdehyde", Encyclopedia of Reagents for Organic Synthesis, 2008, John Wiley & Sons, New York. doi:10.1002/047084289X.rm013.pub2 Article Online Posting Date: March 14, 2008
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.