 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butane-1,2-diol | |
| Other names
1,2-Dihydroxybutane α-Butylene glycol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.663 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C4H10O2 | |
| Molar mass | 90.121 g/mol |
| Density | 1.0023 g/cm3 (20 °C) |
| Melting point | −50 °C (−58 °F; 223 K)[note 1] |
| Boiling point | 195 to 196.9 °C (383.0 to 386.4 °F; 468.1 to 470.0 K) (96.5 °C at 10 mmHg) |
| miscible | |
| Solubility | soluble in ethanol, acetone; sparingly soluble in esters and ethers; insoluble in hydrocarbons |
Refractive index (nD) |
1.4378 (20 °C) |
| Viscosity | 7.3 mPa·s (20 °C) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−532.8 kJ/mol [2] |
Std enthalpy of combustion (ΔcH⦵298) |
−2479 kJ/mol |
| Hazards[3] | |
| Flash point | 90 °C (194 °F; 363 K) |
| Safety data sheet (SDS) | ICSC 0395 |
| Related compounds | |
Related butanediols |
1,3-Butanediol 1,4-Butanediol 2,3-Butanediol |
Related compounds |
Ethylene glycol Propylene glycol 2-Hydroxybutyraldehyde 2-Hydroxybutyric acid α-Ketobutyric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
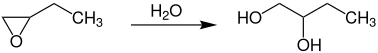
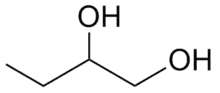
1,2-Butanediol is the organic compound with the formula HOCH2(HO)CHCH2CH3. It is classified as a vic-diol (glycol). It is chiral, although typically it is encountered as the racemic mixture. It is a colorless liquid.
Preparation
This diol was first described by Charles-Adolphe Wurtz in 1859.[4]
It is produced industrially by hydration of 1,2-epoxybutane.[5][6]
This process requires a ten- to twenty-fold excess of water to suppress the formation of polyethers. Depending on the amount of excess water, the selectivity varies from 70 to 92%.[7] Sulfuric acid or strongly acidic ion exchange resins may be used as catalysts, which allows the reaction to occur under 160 °C and at slightly above atmospheric pressure.
1,2-Butanediol is a byproduct of the production of 1,4-butanediol from butadiene.[8] It is also a byproduct of the catalytic hydrocracking of starches and sugars such as sorbitol to ethylene glycol and propylene glycol.[9]
It can also be obtained from the dihydroxylation of but-1-ene by OsO4.
Applications
It has been patented for the production of polyester resins and plasticizers.[6][8] It is a potential feedstock for the industrial production of α-ketobutyric acid, a precursor to some amino acids.[10]
Safety
Notes
- ↑ The value of −50 °C for the melting point is taken from Ullmann's Encyclopedia of Industrial Chemistry and used by the Hazardous Substances Data Bank and the OECD Screening Information Dataset. Other reported values of the melting point range from −114 °C to −30 °C.
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-190. ISBN 0-8493-0462-8..
- ↑ Moureu, H.; Dode, M. (1937), "Chaleurs de formation de l'oxyde d'ethylene, de l'ethanediol et de quelques homologues", Bull. Soc. Chim. Fr., 4: 637–47.
- ↑ 1,2-Butanediol, International Chemical Safety Card 0395, Geneva: International Programme on Chemical Safety, March 1996.
- ↑ Wurtz, A. (1859), "Mémoire sur les glycols ou alcools diatomique" [Dissertation on glycols, or diatomic alcohols], Ann. Chim. Phys., 55: 400.
- 1 2 "Butanediols, Butenediol, and Butynediol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. 2005. doi:10.1002/14356007.a04_455. ISBN 978-3527306732.
- 1 2 1,2-Butanediol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, February 1995.
- ↑ Gräfje, Heinz; Körnig, Wolfgang; Weitz, Hans-Martin; Reiß, Wolfgang; Steffan, Guido; Diehl, Herbert; Bosche, Horst; Schneider, Kurt; Kieczka, Heinz (2019-07-23), "Butanediols, Butenediol, and Butynediol", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, pp. 1–12, doi:10.1002/14356007.a04_455.pub2, ISBN 978-3-527-30673-2, retrieved 2022-02-18
- 1 2 US 4596886, Hasegawa, Ryuichi & Hayashi, Kohji, "Polyester containing impure 1,2-butanediol", published 1986-06-24, assigned to Mitsubishi Monsanto Chemical Company.
- ↑ US 4966658, Berg, Lloyd, "Recovery of ethylene glycol from butanediol isomers by azeotropic distillation", published 1990-10-30. US 5423955, Berg, Lloyd, "Separation of propylene glycol from 1,2-butanediol by azeotropic distillation", published 1995-06-13.
- ↑ US 5155263, Imanari, Makoto; Iwane, Hiroshi & Suzuki, Masashi et al., "Process for preparing α-ketobutyric acid", published 1992-10-13, assigned to Mitsubishi Petrochemical Co., Ltd..