 | |
 | |
| Names | |
|---|---|
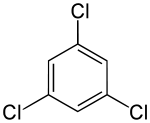
| Preferred IUPAC name
1,3,5-Trichlorobenzene | |
| Other names
sym-Trichlorobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| 1635233 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.281 |
| EC Number |
|
| 601358 | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C6H3Cl3 | |
| Molar mass | 181.44 g·mol−1 |
| Appearance | White solid |
| Melting point | 63 °C (145 °F; 336 K) |
| Boiling point | 208.0 °C (406.4 °F; 481.1 K)[2] |
| 0.6 mg/100 mL | |
| Related compounds | |
Related compounds |
1,2,3-Trichlorobenzene 1,2,4-Trichlorobenzene |
| Hazards[3] | |
| GHS labelling: | |
 | |
| Warning | |
| H302, H312, H315, H319, H332, H335, H412 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| Flash point | 107 °C (225 °F; 380 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
800 mg/kg (oral, rat) 3350 mg/kg (oral, mouse) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,3,5-Trichlorobenzene is an organochlorine compound. It is one of the three isomers of trichlorobenzene. Being more symmetrical than the other isomers, it exists as colourless crystals whereas the other isomers are liquids at room temperature.
It is not formed upon chlorination of benzene. Instead it is prepared by the Sandmeyer reaction from 3,5-dichloroaniline.[4]
See also
- Chlorobenzenes—different numbers of chlorine substituents and isomeric forms
References
- ↑ 1,3,5-Trichlorobenzene, International Programme on Chemical Safety
- ↑ Jaw, Ching-Guang; Chen, I-Ming; Yen, Jui-Hung; Wang, Yei-Shung (December 1999). "Partial solubility parameters of chlorobenzene and chlorophenol compounds at equilibrium distribution in two immiscible phases". Chemosphere. 39 (15): 2607–2620. Bibcode:1999Chmsp..39.2607J. doi:10.1016/s0045-6535(99)00173-3. ISSN 0045-6535.
- ↑ MSDS for 1,3,5-Trichlorobenzene
- ↑ U. Beck; E. Löser (2012). "Chlorinated Benzenes and Other Nucleus-Chlorinated Aromatic Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.o06_o03. ISBN 978-3527306732.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.