| |||
| |||
| Names | |||
|---|---|---|---|
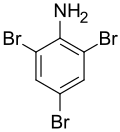
| Preferred IUPAC name
2,4,6-Tribromoaniline | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.005.183 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H4Br3N | |||
| Molar mass | 329.817 g·mol−1 | ||
| Melting point | 120 °C (248 °F; 393 K) | ||
| Boiling point | 300 °C (572 °F; 573 K) | ||
| Insoluble | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Harmful, Corrosive, Toxic | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H301, H302, H311, H312, H315, H318, H319, H331, H332, H373 | |||
| P260, P261, P264, P270, P271, P280, P301+P310, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P310, P311, P312, P314, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Non-flammable | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
2,4,6-Tribromoaniline is a chemical compound with a formula of C6H4Br3N. It is used in organic synthesis of pharmaceuticals, agrochemicals and fire-extinguishing agents. [1]
Synthesis
2,4,6-Tribromoaniline can be prepared by treating bromine water with aniline in a solution of acetic acid or dilute hydrochloric acid:[1]
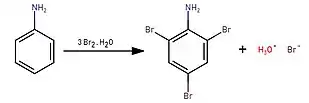 By reacting bromine with aniline in water, a white precipitate immediately forms and that is 2,4,6-tribromoaniline
By reacting bromine with aniline in water, a white precipitate immediately forms and that is 2,4,6-tribromoaniline
See also
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.