 | |
| Names | |
|---|---|
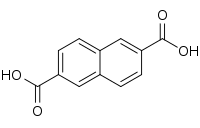
| Preferred IUPAC name
Naphthalene-2,6-dicarboxylic acid | |
| Other names
2,6-Naphthalenedicarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.206 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H8O4 | |
| Molar mass | 216.192 g·mol−1 |
| Appearance | colorless solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2,6-Naphthalenedicarboxylic acid is an organic compound with the formula C10H6(CO2H)2. This colorless solid is one of several isomers of naphthalenedicarboxylic acid. It is a precursor to the high performance polyester polyethylene naphthalate (PEN, poly(ethylene-2,6-naphthalene dicarboxylate)).[1] It is also used in the synthesis of some metal-organic frameworks.
Preparation
The conjugate base of 2,6-naphthalenedicarboxylic acid, when heated, isomerizes to the 1,6-isomer, which is readily converted to 1,6-naphthalenedicarboxylic acid.[2] It is also produced by oxidation of 2,6-diisopropylnaphthalene.[3]
References
- ↑ Lillwitz, L. D. (2001). "Production of Dimethyl-2,6-Naphthalenedicarboxylate: Precursor to Polyethylene Naphthalate". Applied Catalysis A: General. 221 (1–2): 337–358. doi:10.1016/S0926-860X(01)00809-2.
- ↑ Raecke, Bernhard; Schirp, Hubert (1960). "2,6-Naphthalenedicarboxylic acid". Org. Synth. 40: 71. doi:10.15227/orgsyn.040.0071.
- ↑ Gerd Collin; Hartmut Höke; Helmut Greim (2003). "Naphthalene and Hydronaphthalenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. ISBN 978-3527306732..
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.