 | |
| Names | |
|---|---|
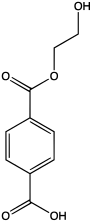
| Preferred IUPAC name
4-[(2-Hydroxyethoxy)carbonyl]benzoic acid | |
| Other names
1,4-Benzenedicarboxylic acid, 1-(2-hydroxyethyl) ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10O5 | |
| Molar mass | 210.185 g·mol−1 |
| Appearance | White solid |
| Melting point | 183–186 °C (361–367 °F; 456–459 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Hydroxyethyl terephthalic acid is an organic compound with the formula HOC2H4O2CC6H4CO2H. It is the monoester of terephthalic acid and ethylene glycol. The compound is a precursor to poly(ethylene terephthalate) (PET), a polymer that is produced on a large scale industrially.[1] 2-Hydroxyethyl terephthalic acid is a colorless solid that is soluble in water and polar organic solvents. Near neutral pH, 2-hydroxyethyl terephthalic acid converts to 2-hydroxyethyl terephthalate, HOC2H4O2CC6H4CO2−.
Occurrence and reactions
2-Hydroxyethyl terephthalic acid is an intermediate in both the formation and hydrolysis of PET. It is produced on a massive scale as the first intermediate in certain routes to PET. Specifically, it is produced in the course of the thermal condensation of terephthalic acid and ethylene glycol:
- HOC2H4OH + HO2CC6H4CO2H → HOC2H4O2CC6H4CO2H + H2O
Further dehydration of 2-hydroxyethyl terephthalic acid gives PET.
It is also produced by the partial hydrolysis of PET, as catalyzed by the enzyme PETase:[2]
- H[O2CC6H4CO2C2H4]nOH + (n−1) H2O → n HO2CC6H4CO2C2H4OH
References
- ↑ Sheehan, Richard J. "Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a26_193. ISBN 978-3527306732.
- ↑ Yoshida, Shosuke; Hiraga, Kazumi; Takehana, Toshihiko; Taniguchi, Ikuo; Yamaji, Hironao; Maeda, Yasuhito; Toyohara, Kiyotsuna; Miyamoto, Kenji; Kimura, Yoshiharu (2016-03-11). "A bacterium that degrades and assimilates poly(ethylene terephthalate)". Science. 351 (6278): 1196–1199. Bibcode:2016Sci...351.1196Y. doi:10.1126/science.aad6359. ISSN 0036-8075. PMID 26965627. S2CID 31146235.