 | |
| Names | |
|---|---|
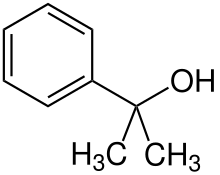
| IUPAC name
2-Phenyl-2-propanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.582 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H12O | |
| Molar mass | 136.19 g/mol |
| Appearance | White to pale yellow odorless solid[1] |
| Density | 0.973 g/cm3[1] |
| Melting point | 28–32 °C (301–305 K)[1] |
| Boiling point | 202[1] °C (396 °F; 475 K) |
| Practically insoluble[2] | |
| Solubility | Soluble in ethanol and benzene[3] |
Refractive index (nD) |
1.49146 (20 °C)[4] |
| Hazards | |
| GHS labelling: | |
 [1] [1] | |
| Danger | |
| H302, H315, H319 | |
| P280, P301+P330+P331, P302+P352, P305+P351+P338, P362[1] | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4300mg/kg (rabbit,transdermal);[1] 1300mg/kg (rat, oral) [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Phenyl-2-propanol is a chemical compound that belongs to the alcohol group.
Synthesis
2-Phenyl-2-propanol can be synthesized through Grignard reaction between phenylmagnesium bromide and acetone.[5][6]
Properties
2-Phenyl-2-propanol is a white to pale-yellow, odorless solid which is combustible yet difficult to ignite and barely dissolves in water.[1][2]
Applications
2-Phenyl-2-propanol can either be applied in organic syntheses, or be reactants or intermediates in agrochemical, medical, and dyestuff fields.[2]
2-Phenyl-2-propanol is the main metabolite of cumene, and therefore 2-phenyl-2-propanol can serve as a biomarker of cumene.[7]
Hazards
According to a report of Federal Institute for Risk Assessment in 2008, there is strong evidence that 2-phenyl-2-propanol may lead to allergic reactions for humans.[8]
References
- 1 2 3 4 5 6 7 8 9 Record of 2-Phenyl-2-propanol in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2017-04-16.
- 1 2 3 2-Phenyl-2-propanol, 98+% at AlfaAesar, accessed on 2017-04-16 (PDF) (JavaScript required).
- ↑ Gail Vance Civille, B. Thomas Carr (2015), Sensory Evaluation Techniques, Fifth Edition, CRC Press, p. 204, ISBN 978-1-4822-0867-2
- ↑ Sigma-Aldrich Co., 2-Phenyl-2-propanol, 97%.
- ↑ William Brown; Christopher Foote; Brent Iverson; Eric Anslyn (2008), Organic Chemistry, Cengage Learning, p. 568, ISBN 978-0-495-38857-9
- ↑ Albany College of Pharmacy and Health Sciences: Preparation of 2-Phenyl-2-propanol (Grignard Reaction), retrieved 16 April 2017.
- ↑ Knecht, U. (2002), "2-Phenyl-2-propanol in Urin [Biomonitoring Methods in German language, 2012]", The MAK-Collection for Occupational Health and Safety, Wiley-VCH Verlag & Co. KGaA, pp. 1–10, doi:10.1002/3527600418.bi9882d0020, ISBN 978-3-527-60041-0
- ↑ BfR: Verbraucher sollten Plastik-Clogs mit starkem Geruch meiden, Stellungnahme Nr. 047/2008 des BfR of 5 November 2008.