 | |
| Names | |
|---|---|
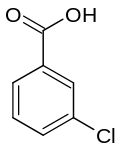
| Preferred IUPAC name
3-Chlorobenzoic acid | |
| Other names
m-Chlorobenzoic acid meta-Chlorobenzoic acid 3BZ | |
| Identifiers | |
3D model (JSmol) |
|
| 907218 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.837 |
| EC Number |
|
| 3664 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5ClO2 | |
| Molar mass | 156.57 g·mol−1 |
| Appearance | white solid |
| Density | 1.517 g/cm3 |
| Melting point | 154 °C (309 °F; 427 K) |
| Boiling point | 275 °C (527 °F; 548 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Flash point | 150 °C (302 °F; 423 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3-Chlorobenzoic acid is an organic compound with the molecular formula ClC6H4CO2H. It is a white solid that is soluble in some organic solvents and in aqueous base.[1]
Synthesis and occurrence
3-Chlorobenzoic acid is prepared by oxidation of 3-chlorotoluene.
References
- ↑ Takao Maki; Kazuo Takeda (2002). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555. ISBN 3527306730..
- ↑ National Center for Biotechnology Information. "3-chlorobenzoic acid". PubChem. Retrieved 27 October 2018.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.