 | |||
| |||
| Names | |||
|---|---|---|---|
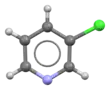
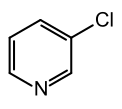
| Preferred IUPAC name
3-Chloropyridine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.009.960 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H4ClN | |||
| Molar mass | 113.54 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 1.194 g/cm3 | ||
| Boiling point | 148 °C (298 °F; 421 K) | ||
Refractive index (nD) |
1.533 | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Warning | |||
| H226, H302, H312, H315, H332 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P312, P321, P322, P330, P332+P313, P362, P363, P370+P378, P403+P235, P501 | |||
| Flash point | 65 °C (149 °F; 338 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
3-Chloropyridine is an organohalide with the formula C5H4ClN. It is a colorless liquid that is mainly used as a building block in organic synthesis.[1]
The compound is a substrate for many coupling processes including the Heck reaction,[2] Suzuki reaction,[3] and Ullmann reaction.[4]
Related compounds
References
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399. ISBN 978-3527306732.
- ↑ Littke, Adam F.; Fu, Gregory C. (2001). "A Versatile Catalyst for Heck Reactions of Aryl Chlorides and Aryl Bromides under Mild Conditions". Journal of the American Chemical Society. 123 (29): 6989–7000. doi:10.1021/ja010988c. PMID 11459477.
- ↑ Littke, Adam F.; Dai, Chaoyang; Fu, Gregory C. (2000). "Versatile Catalysts for the Suzuki Cross-Coupling of Arylboronic Acids with Aryl and Vinyl Halides and Triflates under Mild Conditions". Journal of the American Chemical Society. 122 (17): 4020–4028. doi:10.1021/ja0002058.
- ↑ Alonso, Diego A.; Nájera, Carmen; Pacheco, M Carmen (2002). "Highly Active Oxime-Derived Palladacycle Complexes for Suzuki−Miyaura and Ullmann-Type Coupling Reactions". The Journal of Organic Chemistry. 67 (16): 5588–5594. doi:10.1021/jo025619t. PMID 12153256.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.