.png.webp) | |
| Names | |
|---|---|
| Other names
1-azabicyclo[2.2.2]octan-3-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.989 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H11NO | |
| Molar mass | 125.171 g·mol−1 |
| Hazards | |
| GHS labelling:[1] | |
  | |
| Warning | |
| H302, H312, H332, H411 | |
| P261, P264, P270, P271, P273, P280, P301+P317, P302+P352, P304+P340, P317, P321, P330, P362+P364, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
3-Quinuclidinone is a bicyclic organic compounds with chemical formula HC(C2H4)2(C(O)CH2)N. Its basicity is indicated by the pKa of the conjugate acid, which is 7.2. In contrast quinuclidine is about 100x more basic.[2]
Synthesis and reactions
Its hydrochloride salt can be synthesized by a Dieckman condensation:[3] It is a precursor to quinuclidine.
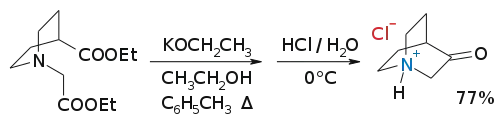
3-quinuclidone hydrochloride synthesis from 1-Carbethoxymethyl-4-carbethoxypiperidine
Organic reduction of 3-quinuclidone gives the compound quinuclidine, structurally related to DABCO, which has one additional bridgehead nitrogen atom.
References
- ↑ "Quinuclidin-3-one". pubchem.ncbi.nlm.nih.gov.
- ↑ Aggarwal, Varinder K.; Emme, Ingo; Fulford, Sarah Y. (2003). "Correlation between pKa and Reactivity of Quinuclidine-Based Catalysts in the Baylis−Hillman Reaction: Discovery of Quinuclidine as Optimum Catalyst Leading to Substantial Enhancement of Scope". The Journal of Organic Chemistry. 68 (3): 692–700. doi:10.1021/jo026671s. PMID 12558387.
- ↑ H. U. Daeniker, C. A. Grob (1964). "3-Quinuclidone Hydrochloride". Organic Syntheses. 44: 86. doi:10.15227/orgsyn.044.0086.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.