In the field of biochemistry, PDPK1 refers to the protein 3-phosphoinositide-dependent protein kinase-1, an enzyme which is encoded by the PDPK1 gene in humans.[5] It is implicated in the development and progression of melanomas.[6]
Function
PDPK1 is a master kinase, which is crucial for the activation of AKT/PKB and many other AGC kinases including PKC, S6K, SGK. An important role for PDPK1 is in the signaling pathways activated by several growth factors and hormones including insulin signaling.
Mice lacking PDPK1 die during early embryonic development, indicating that this enzyme is critical for transmitting the growth-promoting signals necessary for normal mammalian development.
Mice that are deficient in PDPK1 have a ≈40% decrease in body mass, mild glucose intolerance, and are resistant to cancer brought about by hyperactivation of the PI3K pathway (PTEN+/-).[7] [8]
Plant PDK1 plays an important role in regulating PIN-mediated auxin transport, and is thus involved in various developmental processes, such as embryonic development, lateral root formation, vasculature patterning, apical hook formation, gravitropism and phototropism.[9]
Etymology
PDPK1 stands for 3-phosphoinositide-dependent protein kinase 1. PDPK1 functions downstream of PI3K through PDPK1's interaction with membrane phospholipids including phosphatidylinositols, phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate. PI3K indirectly regulates PDPK1 by phosphorylating phosphatidylinositols which in turn generates phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate. However, PDPK1 is believed to be constitutively active and does not always require phosphatidylinositols for its activities.
Phosphatidylinositols are only required for the activation at the membrane of some substrates including AKT. PDPK1 however does not require membrane lipid binding for the efficient phosphorylation of most of its substrates in the cytosol (not at the cell membrane).
Structure
The structure of PDPK1 can be divided into two domains; the kinase or catalytic domain and the PH domain. The PH domain functions mainly in the interaction of PDPK1 with phosphatidylinositol (3,4)-bisphosphate and phosphatidylinositol (3,4,5)-trisphosphate which is important in localization and activation of some of membrane associated PDPK1's substrates including AKT.
The kinase domain has three ligand binding sites; the substrate binding site, the ATP binding site, and the docking site (also known as PIF pocket). Several PDPK1 substrates including S6K and Protein kinase C, require the binding at this docking site. Small molecule allosteric activators of PDPK1 were shown to selectively inhibit activation of substrates that require docking site interaction. These compounds do not bind to the active site and allow PDPK1 to activate other substrates that do not require docking site interaction. PDPK1 is constitutively active and at present, there is no known inhibitor proteins for PDPK1.
The activation of PDPK1's main effector, AKT, is believed to require a proper orientation of the kinase and PH domains of PDPK1 and AKT at the membrane.
Interactions
Phosphoinositide-dependent kinase-1 has been shown to interact with:
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000140992 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000024122 - Ensembl, May 2017
- ↑ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ↑ "Entrez Gene: PDPK1".
- ↑ Scortegagna M, Ruller C, Feng Y, Lazova R, Kluger H, Li JL, De SK, Rickert R, Pellecchia M, Bosenberg M, Ronai ZA (2014). "Genetic inactivation or pharmacological inhibition of Pdk1 delays development and inhibits metastasis of Braf(V600E)::Pten(-/-) melanoma". Oncogene. 33 (34): 4330–9. doi:10.1038/onc.2013.383. PMC 3955742. PMID 24037523.
- ↑ Mora A, Komander D, van Aalten DM, Alessi DR (April 2004). "PDK1, the master regulator of AGC kinase signal transduction". Semin. Cell Dev. Biol. 15 (2): 161–70. doi:10.1016/j.semcdb.2003.12.022. PMID 15209375.
- ↑ Frödin M, Antal TL, Dümmler BA, Jensen CJ, Deak M, Gammeltoft S, Biondi RM (October 2002). "A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation". EMBO J. 21 (20): 5396–407. doi:10.1093/emboj/cdf551. PMC 129083. PMID 12374740.
- ↑ Tan, Shutang; Zhang, Xixi; Kong, Wei; Yang, Xiao-Li; Molnár, Gergely; Vondráková, Zuzana; Filepová, Roberta; Petrášek, Jan; Friml, Jiří; Xue, Hong-Wei (2020). "The lipid code-dependent phosphoswitch PDK1–D6PK activates PIN-mediated auxin efflux in Arabidopsis". Nature Plants. 6 (5): 556–569. doi:10.1038/s41477-020-0648-9. PMID 32393881. S2CID 218593545.
- ↑ Barry FA, Gibbins JM (April 2002). "Protein kinase B is regulated in platelets by the collagen receptor glycoprotein VI". J. Biol. Chem. 277 (15): 12874–8. doi:10.1074/jbc.M200482200. PMID 11825911.
- ↑ Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, Yan J, Sanghera J, Walsh MP, Dedhar S (July 2001). "Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343". J. Biol. Chem. 276 (29): 27462–9. doi:10.1074/jbc.M102940200. PMID 11313365.
- 1 2 3 Hodgkinson CP, Sale GJ (January 2002). "Regulation of both PDK1 and the phosphorylation of PKC-zeta and -delta by a C-terminal PRK2 fragment". Biochemistry. 41 (2): 561–9. doi:10.1021/bi010719z. PMID 11781095.
- 1 2 3 4 Balendran A, Biondi RM, Cheung PC, Casamayor A, Deak M, Alessi DR (July 2000). "A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Czeta (PKCzeta ) and PKC-related kinase 2 by PDK1". J. Biol. Chem. 275 (27): 20806–13. doi:10.1074/jbc.M000421200. PMID 10764742.
- ↑ Biondi RM, Cheung PC, Casamayor A, Deak M, Currie RA, Alessi DR (March 2000). "Identification of a pocket in the PDK1 kinase domain that interacts with PIF and the C-terminal residues of PKA". EMBO J. 19 (5): 979–88. doi:10.1093/emboj/19.5.979. PMC 305637. PMID 10698939.
- 1 2 Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (June 1999). "Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway". EMBO J. 18 (11): 3024–33. doi:10.1093/emboj/18.11.3024. PMC 1171384. PMID 10357815.
- ↑ Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (September 1998). "Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1". Science. 281 (5385): 2042–5. doi:10.1126/science.281.5385.2042. PMID 9748166.
- 1 2 Chun J, Kwon T, Lee E, Suh PG, Choi EJ, Sun Kang S (October 2002). "The Na(+)/H(+) exchanger regulatory factor 2 mediates phosphorylation of serum- and glucocorticoid-induced protein kinase 1 by 3-phosphoinositide-dependent protein kinase 1". Biochem. Biophys. Res. Commun. 298 (2): 207–15. doi:10.1016/s0006-291x(02)02428-2. PMID 12387817.
- ↑ Sato S, Fujita N, Tsuruo T (October 2002). "Regulation of kinase activity of 3-phosphoinositide-dependent protein kinase-1 by binding to 14-3-3". J. Biol. Chem. 277 (42): 39360–7. doi:10.1074/jbc.M205141200. PMID 12177059.
Further reading
- Vanhaesebroeck B, Alessi DR (2000). "The PI3K-PDK1 connection: more than just a road to PKB". Biochem. J. 346 Pt 3 (3): 561–76. doi:10.1042/0264-6021:3460561. PMC 1220886. PMID 10698680.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P (1997). "Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha". Curr. Biol. 7 (4): 261–9. doi:10.1016/S0960-9822(06)00122-9. PMID 9094314. S2CID 2094414.
- Moser BA, Dennis PB, Pullen N, Pearson RB, Williamson NA, Wettenhall RE, Kozma SC, Thomas G (1997). "Dual requirement for a newly identified phosphorylation site in p70s6k". Mol. Cell. Biol. 17 (9): 5648–55. doi:10.1128/MCB.17.9.5648. PMC 232413. PMID 9271440.
- Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, Ashworth A, Bownes M (1997). "3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase". Curr. Biol. 7 (10): 776–89. doi:10.1016/S0960-9822(06)00336-8. PMID 9368760. S2CID 1167390.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Meier R, Alessi DR, Cron P, Andjelković M, Hemmings BA (1997). "Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta". J. Biol. Chem. 272 (48): 30491–7. doi:10.1074/jbc.272.48.30491. PMID 9374542.
- Alessi DR, Kozlowski MT, Weng QP, Morrice N, Avruch J (1998). "3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro" (PDF). Curr. Biol. 8 (2): 69–81. doi:10.1016/S0960-9822(98)70037-5. PMID 9427642. S2CID 18688785.

- Dalby KN, Morrice N, Caudwell FB, Avruch J, Cohen P (1998). "Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK". J. Biol. Chem. 273 (3): 1496–505. doi:10.1074/jbc.273.3.1496. PMID 9430688.
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G (1998). "Phosphorylation and activation of p70s6k by PDK1". Science. 279 (5351): 707–10. Bibcode:1998Sci...279..707P. doi:10.1126/science.279.5351.707. PMID 9445476.
- Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT (1998). "Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B". Science. 279 (5351): 710–4. Bibcode:1998Sci...279..710S. doi:10.1126/science.279.5351.710. PMID 9445477.
- Walker KS, Deak M, Paterson A, Hudson K, Cohen P, Alessi DR (1998). "Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha". Biochem. J. 331 ( Pt 1) (1): 299–308. doi:10.1042/bj3310299. PMC 1219352. PMID 9512493.
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT (1998). "Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B". Curr. Biol. 8 (12): 684–91. doi:10.1016/S0960-9822(98)70274-X. PMID 9637919. S2CID 13936854.
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ (1998). "Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1". Science. 281 (5385): 2042–5. doi:10.1126/science.281.5385.2042. PMID 9748166.
- Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, Cohen P, Alessi DR, Lucocq J (1999). "Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1". Biochem. J. 337 ( Pt 3) (3): 575–83. doi:10.1042/0264-6021:3370575. PMC 1220012. PMID 9895304.
- Kobayashi T, Cohen P (1999). "Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2". Biochem. J. 339 ( Pt 2) (2): 319–28. doi:10.1042/0264-6021:3390319. PMC 1220160. PMID 10191262.
- Balendran A, Casamayor A, Deak M, Paterson A, Gaffney P, Currie R, Downes CP, Alessi DR (1999). "PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2". Curr. Biol. 9 (8): 393–404. doi:10.1016/S0960-9822(99)80186-9. PMID 10226025. S2CID 16473977.
- Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA (1999). "Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway". EMBO J. 18 (11): 3024–33. doi:10.1093/emboj/18.11.3024. PMC 1171384. PMID 10357815.
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G (1999). "A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans". Genes Dev. 13 (11): 1438–52. doi:10.1101/gad.13.11.1438. PMC 316759. PMID 10364160.
- Casamayor A, Morrice NA, Alessi DR (1999). "Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo". Biochem. J. 342 ( Pt 2) (2): 287–92. doi:10.1042/0264-6021:3420287. PMC 1220463. PMID 10455013.
External links
- 3-phosphoinositide-dependent+protein+kinase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)




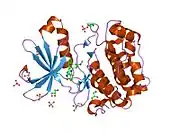
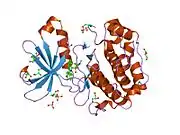


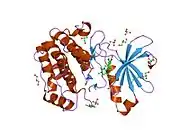

![1z5m: Crystal Structure Of N1-[3-[[5-bromo-2-[[3-[(1-pyrrolidinylcarbonyl)amino]phenyl]amino]-4-pyrimidinyl]amino]propyl]-2,2-dimethylpropanediamide Complexed with Human PDK1](../I/PDB_1z5m_EBI.jpg.webp)