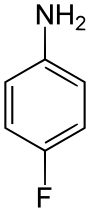
 | |
| Names | |
|---|---|
| Other names
p-Fluoroaniline | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.123 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
| UN number | 2941 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6FN | |
| Molar mass | 111.119 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.1725 g/cm3 |
| Melting point | −1.9 °C (28.6 °F; 271.2 K) |
| Boiling point | 188 °C (370 °F; 461 K) |
| Hazards | |
| GHS labelling:[1] | |
    | |
| Danger | |
| H302, H312, H314, H315, H317, H319, H332, H372, H373, H410, H411 | |
| P260, P261, P264, P264+P265, P270, P271, P272, P273, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P319, P321, P330, P332+P317, P333+P313, P337+P317, P362+P364, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Fluoroaniline is an organofluorine compound with the formula FC6H4NH2. A colorless liquid, it is one of three isomers of fluoroaniline. It is used as a precursor to various potential and real applications.
4-Fluoroaniline can be prepared by the hydrogenation of 4-nitrofluorobenzene.[2]
It is a common building block in medicinal chemistry and related fields.[3] For example, it is a precursor to the fungicide fluoroimide. It has also been evaluated for the production of ligands for homogeneous catalysis.[4]
References
- ↑ "4-Fluoroaniline". pubchem.ncbi.nlm.nih.gov.
- ↑ Jagadeesh, Rajenahally V.; Surkus, Annette-Enrica; Junge, Henrik; Pohl, Marga-Martina; Radnik, Jörg; Rabeah, Jabor; Huan, Heming; Schünemann, Volker; Brückner, Angelika; Beller, Matthias (2013). "Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines". Science. 342 (6162): 1073–1076. Bibcode:2013Sci...342.1073J. doi:10.1126/science.1242005. PMID 24288327. S2CID 11780985.
- ↑ Wei, Jinbao; Chen, Jinghong; Ju, Peijun; Ma, Le; Chen, Li; Ma, Weidong; Zheng, Tao; Yang, Guangyi; Wang, Yong-Xiang (2019). "Synthesis and Biological Evaluation of 4β-N-Acetylamino Substituted Podophyllotoxin Derivatives as Novel Anticancer Agents". Frontiers in Chemistry. 7: 253. Bibcode:2019FrCh....7..253W. doi:10.3389/fchem.2019.00253. PMC 6491884. PMID 31106192.
- ↑ Mitani, Makoto; Mohri, Jun-Ichi; Yoshida, Yasunori; Saito, Junji; Ishii, Seiichi; Tsuru, Kazutaka; Matsui, Shigekazu; Furuyama, Rieko; Nakano, Takashi; Tanaka, Hidetsugu; Kojoh, Shin-Ichi; Matsugi, Tomoaki; Kashiwa, Norio; Fujita, Terunori (2002). "Living Polymerization of Ethylene Catalyzed by Titanium Complexes Having Fluorine-Containing Phenoxy−Imine Chelate Ligands". Journal of the American Chemical Society. 124 (13): 3327–3336. doi:10.1021/ja0117581. PMID 11916417.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.