 | |
| Names | |
|---|---|
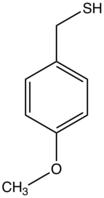
| Preferred IUPAC name
(4-Methoxyphenyl)methanethiol | |
| Other names
p-methoxybenzylmercaptan | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.025.812 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H10OS | |
| Molar mass | 154.23 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 89–94 °C (192–201 °F; 362–367 K) 2.5 mm Hg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Methoxybenzylthiol is an organosulfur compound with the formula CH3OC6H4CH2SH. A colorless, odiferous oil, it is a reagent used as a protected thiol.[1]
References
- ↑ Atwal, Karnail S.; Rovnyak, George C.; O'Reilly, Brian C.; Schwartz, Joseph (1989). "Substituted 1,4-dihydropyrimidines. 3. Synthesis of selectively functionalized 2-hetero-1,4-dihydropyrimidines". The Journal of Organic Chemistry. 54 (25): 5898–5907. doi:10.1021/jo00286a020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.