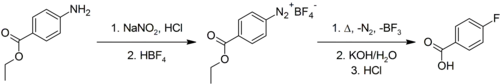| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Fluorobenzoic acid | |||
| Other names
p-fluorobenzoic acid, para-fluorobenzoic acid, 4-fluoro-benzoic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.600 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H5FO2 | |||
| Molar mass | 140.113 g·mol−1 | ||
| Appearance | white solid | ||
| Density | 1.479 g/cm3 | ||
| Melting point | 184 °C (363 °F; 457 K) | ||
| Boiling point | 253.687 °C (488.637 °F; 526.837 K) at 760 mmHg | ||
| 1200 mg/L | |||
| log P | 2.07 | ||
| Acidity (pKa) | 4.14 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Irritates lungs, eyes, skin | ||
| GHS labelling:[1] | |||
  | |||
| Danger | |||
| H302, H318 | |||
| P264, P270, P280, P301+P312, P305+P351+P338, P310, P330, P501 | |||
| Flash point | 107.226 °C (225.007 °F; 380.376 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
4-Fluorobenzoic acid (p-fluorobenzoic acid) is an organic compound with the formula C7H5FO2. This colourless solid is a derivative of benzoic acid carboxylic acid. It is a synthetic intermediate.
Preparation
4-Fluorobenzoic acid is commercially available. It may be prepared via the Schiemann reaction, in which a 4-aminobenzoic acid, protected as the ethyl ester, is diazotised and then fluoride introduced using tetrafluoroborate. Hydrolysis of the ester converts it back to the free acid.[2]
4-Fluorobenzoic acid has been observed to form by the aerobic biotransformation of 4-fluorocinnamic acid.[3]
See also
References
- ↑ GHS: PubChem 9973
- ↑ G. Schiemann; W. Winkelmüller (1943). "p-Fluorobenzoic Acid". Organic Syntheses.; Collective Volume, vol. 2, p. 299
- ↑ Freitas Dos Santos, Luisa M.; Spicq, Arnaud; New, Anthony P.; Lo Biundo, Giuseppe; Wolff, Jean-Claude; Edwards, Andrew (2001). "Aerobic biotransformation of 4-fluorocinnamic acid to 4-fluorobenzoic acid". Biodegradation. 12 (1): 23–9. doi:10.1023/A:1011973824171. PMID 11693292. S2CID 30830147.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.