 | |
| Names | |
|---|---|
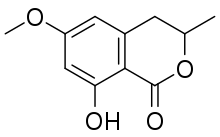
| Preferred IUPAC name
8-Hydroxy-6-methoxy-3-methyl-3,4-dihydro-1H-2-benzopyran-1-one | |
| Other names
8-Hydroxy-6-methoxy-3-methylisochroman-1-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C11H12O4 | |
| Molar mass | 208.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
6-Methoxymellein is a dihydroisocoumarin, a phenolic compound found in carrots and carrot purées. It is responsible for bitterness in carrots.[1][2] It is a phytoalexin, induced in carrot slices by UV-C,[3] that allows resistance to Botrytis cinerea[4] and other microorganisms.[5]
Biosynthesis
6-Methoxymellein is formed from S-adenosyl methionine and 6-hydroxymellein by the enzyme 6-hydroxymellein O-methyltransferase with secondary production of S-adenosylhomocysteine.[6]
References
- ↑ Structural and Sensory Characterization of Compounds Contributing to the Bitter Off-Taste of Carrots (Daucus carota L.) and Carrot Puree. Andreas Czepa and Thomas Hofmann, J. Agric. Food Chem., 2003, 51, pages 3865-3873, doi:10.1021/jf034085+ PMID 12797757
- ↑ Determination and Distribution of 6-Methoxymellein in Fresh and Processed Carrot Puree by a Rapid Spectrophotometric Assay. S.T. Talcott and L.R. Howard, J. Agric. Food Chem., 1999, 47 (8), pages 3237–3242, doi:10.1021/jf990288f
- ↑ Induction of 6-Methoxymellein and Resistance to Storage Pathogens in Carrot Slices by UV-C. J. Mercier, J. Arul, R. Ponnampalam and M. Boulet, Journal of Phytopathology, Volume 137, Issue 1, pages 44–54, January 1993, doi:10.1111/j.1439-0434.1993.tb01324.x
- ↑ Cell death, 6-methoxymellein accumulation, and induced resistance to Botrytis cinerea in carrot root slices. R. Hoffman and J.B. Heale, Physiological and Molecular Plant Pathology, Volume 30, Issue 1, January 1987, Pages 67–75, doi:10.1016/0885-5765(87)90083-X
- ↑ Isolation and antimicrobial activity of the phytoalexin 6-methoxymellein from cultured carrot cells. Fumiya Kurosaki and Arasuke Nishi, Phytochemistry, Volume 22, Issue 3, 1983, Pages 669–672, doi:10.1016/S0031-9422(00)86959-9
- ↑ "MetaCyc 6-methoxymellein biosynthesis". www.biocyc.org.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.