 | |
| Names | |
|---|---|
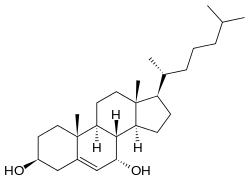
| IUPAC name
Cholest-5-ene-3β,7α-diol | |
| Systematic IUPAC name
(1R,3aS,3bS,4S,7S,9aR,9bS,11aR)-9a,11a-Dimethyl-1-[(2R)-6-methylheptan-2-yl]-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthrene-4,7-diol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | 7+alpha-hydroxycholesterol |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H46O2 | |
| Molar mass | 402.653 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
7α-Hydroxycholesterol is a precursor of bile acids, created by cholesterol 7α-hydroxylase (CYP7A1). Its formation is the rate-determining step in bile acid synthesis.[1]
References
- ↑ Chiang JY, Ferrell JM (March 2020). "Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy". American Journal of Physiology. Gastrointestinal and Liver Physiology. 318 (3): G554–G573. doi:10.1152/ajpgi.00223.2019. PMC 7099488. PMID 31984784.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.