 | |
| Names | |
|---|---|
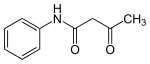
| Preferred IUPAC name
3-Oxo-N-phenylbutanamide | |
| Other names
Acetoacetylaminobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.725 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H11NO2 | |
| Molar mass | 177.203 g·mol−1 |
| Appearance | Colourless solid |
| Melting point | 83 to 88 °C (181 to 190 °F; 356 to 361 K) |
| low | |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H302, H312, H332, H373 | |
| P260, P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P312, P314, P322, P330, P363, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Acetoacetanilide is an organic compound with the formula CH3C(O)CH2C(O)NHC6H5. It is the acetoacetamide derivative of aniline. It is a white solid that is poorly soluble in water. This chemical and many related compounds (prepared from various aniline derivatives) are used in the production of organic pigments called arylide yellows. Acetoacetanilides usually exist as the keto-amide tautomer according to X-ray crystallography.[1]
Preparation and reactions
Acetoacetanilide is prepared by acetoacetylation of aniline using diketene.[2] Many analogues have been prepared.[3]
To make the dyes, acetoacetanilides are coupled to diazonium salts, "azo coupling".[4]
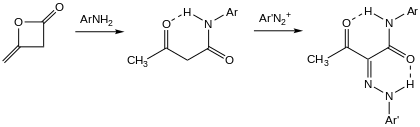
Acetoacetylation with diketene followed by diazo coupling.
In the presence of sulfuric acid, acetoacetanilide dehydrates to give 4-methyl-2-quinolone.[5]
See also
References
- ↑ Gilli, Paola; Bertolasi, Valerio; Ferretti, Valeria; Gilli, Gastone (2000). "Evidence for Intramolecular N−H···O Resonance-Assisted Hydrogen Bonding in β-Enaminones and Related Heterodienes. A Combined Crystal-Structural, IR and NMR Spectroscopic, and Quantum-Mechanical Investigation". Journal of the American Chemical Society. 122 (42). doi:10.1021/ja000921+.
- ↑ Williams, Jonathan W.; Krynitsky, John A. (1941). "Acetoacetanilide". Organic Syntheses. 21: 4. doi:10.15227/orgsyn.021.0004.
- ↑ Jaffe, Edward E. (2004). "Pigments, Organic". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.151807011001060605.a01.pub2. ISBN 978-0-471-48494-3.
- ↑ K. Hunger. W. Herbst "Pigments, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. doi:10.1002/14356007.a20_371
- ↑ Lauer, W. M.; Kaslow, C. E. (1944). "4-Methylcarbostyril". Organic Syntheses. 24: 68. doi:10.15227/orgsyn.024.0068.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
